
Adult Basic Education
Science
Chemistry 1102
Chemical Reactions
Curriculum Guide
Credit Value: 1
Chemistry Concentration
Chemistry 1102
Chemistry 2102A
Chemistry 2102B
Chemistry 2102C
Chemistry 3102A
Chemistry 3102B
Chemistry 3102C

Table of Contents
To the Instructor...............................................................v
Introduction to Chemistry 1102 .............................................v
Curriculum Guides.......................................................v
Study Guides .......................................................... vi
Resources ............................................................. vi
Recommended Evaluation .............................................. vii
Unit 1 - Investigating Chemical Reactions ..................................... Page 2
Unit 2 - Formula Writing ................................................... Page 6
Unit 3 - Equation Writing ................................................. Page 12
Unit 4 - Introduction to Acids and Bases ..................................... Page 14
Appendix A ............................................................ Page 19

Curriculum Guide Chemistry 1102v
To the Instructor
I. Introduction to Chemistry 1102
This is the first course in the ‘Chemistry Concentration’ of the Adult Basic Education
program. It assumes that students already have some understanding of atomic structure
and the periodic table. This is not a safe assumption for most ABE students. You may,
therefore, need to spend some time teaching these concepts before students proceed with
this course.
In this course students will learn about naming and writing formulas for ionic and
molecular compounds. They will also learn to write and balance a variety of equation
types. Students should be expected to show a high level of mastery of these topics in
order to have the necessary foundation to build upon as they continue in the Chemistry
concentration in ABE.
It is very important to note that this course is a pre-requisite to all the other Chemistry
courses.
II. Curriculum Guides
Each new ABE Science course has a Curriculum Guide for the instructor and a Study
Guide for the student. The Curriculum Guide includes the specific curriculum outcomes
for the course. Suggestions for teaching, learning, and assessment are provided to support
student achievement of the outcomes. Each course is divided into units. Each unit
comprises a two-page layout of four columns as illustrated in the figure below. In some
cases the four-column spread continues to the next two-page layout.
Curriculum Guide Organization:
The Two-Page, Four-Column Spread
Unit Number - Unit Title Unit Number - Unit Title
Outcomes
Specific
curriculum
outcomes for
the unit.
Notes for Teaching and
Learning
Suggested activities,
elaboration of outcomes, and
background information.
Suggestions for Assessment
Suggestions for assessing
students’ achievement of
outcomes.
Resources
Authorized and
recommended
resources that
address
outcomes.

Curriculum Guide Chemistry 1102vi
To the Instructor
III. Study Guides
The Study Guide provides the student with the name of the text(s) required for the course
and specifies the sections and pages that the student will need to refer to in order to
complete the required work for the course. It guides the student through the course by
assigning relevant reading and providing questions and/or assigning questions from the
text or some other resource. Sometimes it also provides important points for students to
note. (See the To the Student section of the Study Guide for a more detailed explanation
of the use of the Study Guides.) The Study Guides are designed to give students some
degree of independence in their work. Instructors should note, however, that there is
much material in the Curriculum Guides in the Notes for Teaching and Learning and
Suggestions for Assessment columns that is not included in the Study Guide and
instructors will need to review this information and decide how to include it.
IV. Resources
Essential Resources
Science 10
Nelson Science 10 Teacher’s Resource for Unit 2 - Chemical Processes
Recommended Resources
Science 1206: Curriculum Guide:
http://www.ed.gov.nl.ca/edu/sp/sh/sci/sci1206/unit3.PDF
Nelson Publishing Web Site:
http://www.science.nelson.com
Computerized Assessment Bank for Nelson Science 10, Nelson.
Other Resources
Center for Distance Learning and Innovation: http://www.cdli.ca/

Curriculum Guide Chemistry 1102vii
To the Instructor
V. Recommended Evaluation
Written Notes 10%
Labs/Assignments 20%
Test(s) 20%
Final Exam (entire course) 50%
100%
The overall pass mark for the course is 50%.
Chemical Reactions

Unit 1 - Investigating Chemical Reactions
Curriculum Guide Chemistry 1102Page 2
Outcomes
1.1 Understand that science and
technology are an integral part of
everyday life.
1.1.1 Define chemistry and
matter.
1.1.2 Identify examples of
chemistry and technology in
everyday life.
1.2 Explain how matter is
classified.
1.2.1 Name and define the two
categories of matter (pure
substances and mixtures).
1.2.2 Name and define the two
categories of pure
substances.
1.2.3 Name and define the two
categories of mixtures.
1.3 Evaluate and select
appropriate methods/tests to
investigate the presence of
chemicals.
1.3.1 Explain the difference
between physical and
chemical properties.
Notes for Teaching and Learning
Students who have recently completed Intermediate
Science (Grade 9) should be familiar with the terms and
concepts at the beginning of this unit (Outcomes 1.1 -
1.3). For other students, this material will be new and may
require extra practice for reinforcement.
Students will be introduced to many new terms
throughout this course. Instructors could suggest that
students start a vocabulary list and add to it regularly as
they work through the course.

Unit 1 - Investigating Chemical Reactions
Curriculum Guide Chemistry 1102Page 3
Suggestions for Assessment
Instructors should assess the student’s level of understanding by
reading student answers to questions from the Study Guide and
providing feedback.
Instructors should ensure that all necessary terms are being added
to the student’s vocabulary list and provide students with ideas
about how to successfully remember definitions.
The CDLI (Center for Distance Learning and Innovation) site was
developed for distance delivery of selected high school courses. It
contains lots of useful materials that could be used for
reinforcement in the Adult Basic Education program.
Note: You will need a username and password to enter the CDLI
site.
There are numerous web sites that have instructional materials
that instructors could use to assist their teaching of Chemistry. A
few of these are listed in Resources.
Resources
The center for distance
learning and innovation
website:
http://www.cdli.ca/
Textbook website:
http://www.science.nelson.c
om/
Other useful websites:
www.chemtutor.com
http://www.dbhs.wvusd.k12
.ca.us/webdocs/
Science 10 , Chapter 5,
pages 170 - 182.
Science 10 Teacher’s
Resource, Unit 2,
“Chemical Processes”.
Science 10 Teacher’s
Resource, Applied
Supplement.
Science 10 , Computerized
Assessment Bank.

Unit 1 - Investigating Chemical Reactions
Curriculum Guide Chemistry 1102Page 4
Outcomes
1.3.2 Explain the difference
between physical and
chemical change.
1.3.3 Determine the physical and
chemical properties of
selected substances.
1.3.4 Define electrolyte and
nonelectrolyte.
1.4 Demonstrate a knowledge of
WHMIS standards.
1.4.1 Describe the WHMIS
information system and its
use.
1.4.2 Identify the eight WHMIS
symbols.
1.4.3 Describe the MSDS sheet
and its use.
1.4.4 Identify the nine categories
on a MSDS sheet.
Notes for Teaching and Learning
Students should complete the work for outcome 1.4
before working in the lab. This can be achieved by having
students complete the “WHMIS Activity” found in
Appendix A. Instructors should review with students the
following from the Skills Handbook (page 657): B: Safety
in the Laboratory, Q: Lab Reports, and Figure 3: Common
Laboratory Equipment, before students begin work in the
lab.
Safe practices and proper use of equipment are very
important in the laboratory. For all laboratory activities,
instructors should ensure that students recognize WHMIS
standards.
Any chemicals purchased will come with an MSDS sheet
that students can investigate. One example of an MSDS
sheet is provided in the Appendix.
Lab safety procedures should be reviewed with students
before they begin work in the lab. Instructors should use
Skills Handbook, A, “Safety Conventions and Symbols”,
and B, “Safety in the Laboratory” for a review of safety.
Outcome 1.3.3 can be achieved by carrying out
Investigation 5.3, “Testing Properties of Substances”.
Instructors should review the relevant sections of the
Skills Handbook, K “Planning an Investigation”, with
students before they begin working in the lab.
Instructors should explain to students what is expected to
be submitted for their lab report. Blackline Master 5.3b
could be provided to the students to record their
observations.

Unit 1 - Investigating Chemical Reactions
Curriculum Guide Chemistry 1102Page 5
Suggestions for Assessment
Questions 1.1 - 1.8 in the Study Guide should be assigned to
cover Outcome1.1, 1.2, 1.3.1, and 1.3.2. Students will find the
answers to these questions in the Introduction and Section 1.5 of
the text.
Instructors should assess the student’s level of understanding by
reading student answers to questions from the Study Guide and
providing feedback. Instructors should assign additional practice
and/or review as needed.
The Assignment, “WHMIS Activity”, and the
Laboratory,“Testing Properties of Substances” , from the study
guide, should be assigned marks and used as part of the
evaluation for the course.
Blackline Masters 5.1c, “Safety Symbols”, and 5.1d, “Lab Safety
Concept Map”, could be used for assessment of student’s
knowledge of safety procedures.
Instructors should refer to the Teacher’s Resource for suggestions
and notes for delivery of the lab.
Resources
“WHMIS Activity”,
Appendix A of this guide.
Science 10, Skills
Handbook, K: Planning an
Investigation.
Science 10, pp. 180 -182,
Core Lab #1, “Testing
Properties of Substances”.
Blackline Master
5.1c,”Safety Symbols”.
Blackline Master 5.1d,
“Lab Safety Concept Map”.
Unit 2 - Formula Writing
Curriculum Guide Chemistry 1102Page 6
Outcomes
2.1 Describe the structure of the
periodic table.
2.1.1 Define periodic table.
2.1.2 Define chemical families
and identify the chemical
families (alkali metals,
alkaline earth metals,
halogens, noble gases) in
the periodic table.
2.2 Describe the structure of
atoms and ions.
2.2.1 Name and describe the three
main parts of the atom.
2.2.2 Define ion and explain how
ions are formed.
2.2.3 Draw Bohr diagrams of
atoms and ions.
Notes for Teaching and Learning
Students who have recently completed Intermediate
Science (Grade 9) should be familiar with the terms and
concepts at the beginning of this unit (Outcomes 2.1, 2.2).
For other students, this material will be new and may
require extra practice for reinforcement.
Blackline Master 5.5a, “Periodic Table of Elements”, can
be copied and given to students to use as they work
through the remainder of this course.
The Teacher’s Resource has a good explanation of the
concepts in Outcomes 2.1 and 2.2 in the background
information for Section 5.5.
The answers for all questions from the text can be found
in the Teacher’s Resource.

Unit 2 - Formula Writing
Curriculum Guide Chemistry 1102Page 7
Suggestions for Assessment
Questions 2.1 - 2.5 in the Study Guide should be assigned to
cover Outcomes 2.1. and 2.2. Students will find the answers to
these questions in Section 5.5 of the text.
Activity 5.7 “Ionic Charges and Chemical Families”, pages 190 -
191, may be assigned and used for assessment of students’
understanding of the kinds of ions formed by elements of the
major chemical families and their ionic charges.
Blackline Master 5.5b, “Elements, Compounds, and the Periodic
Table Crossword”, can be used for assessment of this section.
Resources
Science 10, Chapter 5,
pages 184 - 214.
Blackline Master 5.5a,
“Periodic Table of
Elements”.
Blackline Master 5.5b,
“Elements, Compounds,
and the Periodic Table
Crossword”.

Unit 2 - Formula Writing
Curriculum Guide Chemistry 1102Page 8
Outcomes
2.3 Describe the usefulness of
IUPAC scientific nomenclature
system to convey chemical
information.
2.3.1 Define molecule,
compound, molecular
element (diatomic
molecule), molecular
formula, empirical formula.
2.3.2 Differentiate between ionic
and molecular compounds.
2.4 Name and write formulas for
some common ionic compounds
(both binary and complex).
2.4.1 Define valence.
2.4.2 Define polyatomic ion.
2.4.3 Using IUPAC rules,
determine the formulas for
common ionic compounds
(including simple and
polyatomic ions, ions that
can form multiple charges,
and ionic hydrates) .
Notes for Teaching and Learning
Students who have recently completed Intermediate
Science (Grade 9) should be familiar with the terms
covered in outcome 2.3.1. For other students, this material
will be new and may require extra practice for
reinforcement.
It is also important to note that the terms in outcome
2.3.1. are not defined in the text and students will need to
be provided with these definitions. These are covered in
Handout 2 - “Introduction to IUPAC”, found in Appendix
A of this guide.
Students need lots of practice with naming and writing
formulas for ionic compounds. Instructors should assess
student needs and provide worksheets as necessary.
The explanations in the text for naming ionic compounds
of different types are very brief.
Naming ionic hydrates is not covered in the text.
Instructors should use Handout 2 - “Naming Ionic
Hydrates”, in Appendix A, to teach this topic and provide
students with the worksheet in Handout 3 for practice.
This topic is important for chemistry courses that follow.
NOTE: A periodic table of ions as well as a table of
polyatomic ions should be provided to students.

Unit 2 - Formula Writing
Curriculum Guide Chemistry 1102Page 9
Suggestions for Assessment
Questions 2.8 - 2.12 in the Study Guide should be assigned to
cover Outcome 2.4. Students will find the answers to some of
these questions in Sections 5.8 and 5.9 of the text.. They will also
need to refer to Handout 3 (found in Appendix A of their Study
Guide).
Blackline Masters 5.8 “Ionic Compounds: Names and Formulas
Worksheet”, and 5.9, “Polyatomic Compounds: Names and
Formulas Worksheet”, can be used to assess students’
understanding of naming ionic compounds.
The Nelson Science website has links that students can access for
additional practice. There are also many computer software
programs that can be used to provide extra practice.
Resources
Blackline Master 5.8,
“Ionic Compounds: Names
and Formulas Worksheet”.
Blackline Master 5.9,
“Polyatomic Compounds:
Names and Formulas
Worksheet”.
Handout 3 - “Naming Ionic
Hydrates”, in Appendix A.

Unit 2 - Formula Writing
Curriculum Guide Chemistry 1102Page 10
Outcomes
2.4.4 Using IUPAC rules,
determine the names of
ionic compounds (including
simple and polyatomic ions,
ions that can form multiple
charges, and ionic hydrates).
2.5 Name and write formulas for
common molecular compounds,
including the use of prefixes.
2.5.1 Define covalent bond.
2.5.2 Using IUPAC rules,
determine the names of
binary molecular
compounds.
2.5.3 Using IUPAC rules,
determine the formulas of
binary molecular
compounds.
2.5.4 Write formulas for several
common molecular
compounds using trivial
(common) names.
Notes for Teaching and Learning
Students need lots of practice with naming and writing
formulas for molecular compounds. Instructors should
assess student needs and provide worksheets as necessary.
A summary of rules for naming compounds and writing
formulas is found in the handout , “IUPAC Naming of
Compounds and Writing Formulas”, in Appendix A.
Instructors should ensure that students review it carefully
and complete the worksheets that are included. Many
computer software programs are available for additional
practice.

Unit 2 - Formula Writing
Curriculum Guide Chemistry 1102Page 11
Suggestions for Assessment
Questions 2.13 - 2.14 in the Study Guide should be assigned to
cover Outcome 2.5. Students will find the answers to these
questions in Section 5.11 of the text.
Blackline Master 5.11, “Molecular Compounds: Names and
Formulas Worksheet”, can be used to assess the student’s ability
to apply knowledge and understanding of these concepts.
The worksheets included with the handout , “IUPAC Naming of
Compounds and Writing Formulas”, should be checked by the
instructor to assess the student’s understanding. Extra explanation
and practice should be provided, if needed.
A quiz might be useful here to ascertain if students have mastered
the concepts covered so far. The mark for this quiz may be used
as part of the evaluation of the course.
Resources
Blackline Master 5.11,
“Molecular Compounds:
Names and Formulas
Worksheet”.
Handout 3 - “IUPAC
Naming of Compounds and
Writing Formulas”.
Science 10 , Computerized
Assessment Bank.

Unit 3 - Equation Writing
Curriculum Guide Chemistry 1102Page 12
Outcomes
3.1 Represent chemical reactions
using word equations.
3.1.1 Define word equation.
3.1.2 Write word equations to
represent a variety of
reactions.
3.2 Represent chemical reactions
and the conservation of mass, using
balanced symbolic equations.
3.2.1 Define the Law of
Conservation of mass.
3.2.2 Write and balance reactions
that illustrate a variety of
reaction types; including
combustion, synthesis
(combination),
decomposition, single and
double displacement
(replacement).
3.2.3 Recognize and predict the
products of different types
of chemical reactions;
including combustion,
synthesis, decomposition,
single and double
displacements.
Notes for Teaching and Learning
Instructors should ensure that students understand the
format for writing equations.
Students often don’t realize that the arrow ( 6) signifies
produces or yields and must not be substituted with the
equals sign (=).
Students often try to balance equations by changing the
subscripts in the chemical formulas. Instructors should
emphasize that subscripts in correctly written formulas are
never changed during the balancing process.
Instructors will likely need to work through several
examples of balancing equations with students, in
addition to those in the text.
If possible, have students working in pairs to complete the
core lab. Provide students with Blackline Masters 6.8
and/or 6.9 to record their observations when completing
the lab.
The Teacher’s Resource should be consulted for
information on the lab that is chosen.

Unit 3 - Equation Writing
Curriculum Guide Chemistry 1102Page 13
Suggestions for Assessment
Questions 3.1 - 3.2. in the Study Guide should be assigned to
cover Outcome 3.1. Students will find the answers to these
questions in Section 6.1 of the text.
Questions 3.3 - 3.15 in the Study Guide should be assigned to
cover the rest of the outcomes for this unit. Students will find the
answers to some of these questions in Sections 6.3, 6.5, 6.6, 6.7,
and 6.10 of the text.
Students should also complete and submit a report for either
Investigation 6.8 or 6.9.
Writing and balancing equations is usually difficult for students
and instructors will need to assess their success and assign
additional practice as needed. Chapter 6 Review provides some
additional practice. Other resources should also be used.
Blackline Masters 6.5a, “How to Count Atoms Review” and 6.5b,
“Counting Atoms Worksheet”, should be used to review concepts
and to help students prepare for learning how to balance
equations.
Blackline Master 6.13, “Types of Chemical Reactions
Worksheet”, and Blackline Master Chapter 6 Review, “Chemical
Reactions Word Search”, can be used to assess students’
understanding of the outcomes for Unit 3.
Instructors may give a quiz on Unit 3 to ascertain if students have
mastered the concepts covered in Unit 3. The mark for this quiz
may be used as part of the evaluation of the course.
Resources
Science 10, Chapter 6,
pages 216 -252.
Science 10, pp. 236 - 237,
Core Lab #2, “Putting
Things Together”
or
Science 10, pp. 238 - 239, ,
“Taking Things Apart”.
Blackline Master 6.5a
“How to Count Atoms
Review”.
Blackline Master 6.5b,
“Counting Atoms
Worksheet”.
Blackline Master 6.8,
“Putting Things Together”.
Blackline Master 6.9,
“Taking Things Apart”.
Blackline Master 6.13,
“Types of Chemical
Reactions Worksheet”.
Blackline Master Chapter 6
Review, “Chemical
Reactions Word Search”.

Unit 4 - Introduction to Acids and Bases
Curriculum Guide Chemistry 1102Page 14
Outcomes
4.1 Classify simple acids, bases,
and salts on the basis of their names
and formulas.
4.1.1 Name and write formulas
for some common acids and
bases, using the periodic
table, a list of ions, and
rules for naming acids.
4.1.2 Define acids as molecules
that ionize in water to
produce hydrogen ions (H ).
+
4.1.3 Identify the physical
properties of acids.
4.1.4 Define bases as ionic
compounds that contain the
hydroxide ion (OH ).
-
4.1.5 Define salts as ionic
compounds.
Notes for Teaching and Learning
The text does not explain the details of how to name
acids. The handout, “Naming Acids”, in Appendix A,
covers the naming acids part of outcome 4.1.1. Students
should be provided with a copy of this handout.
There is no need for special instruction for naming bases
and salts. Students will use the rules that they have
already learned for naming compounds.
Students should be provided with worksheets to practice
naming acids and bases. Instructors can find worksheets
on the CDLI site or on numerous other web sites or in
resource packages.
A detailed study of acids, bases, pH and so forth is not
expected at this point.

Unit 4 - Introduction to Acids and Bases
Curriculum Guide Chemistry 1102Page 15
Suggestions for Assessment
Review sheets can be used for assessment of students’ ability to
recognize substances as acids or bases and name them properly.
Resources
Science 10, Chapter 8,
pages 288-289, 293 - 299,
314, 317 - 319.
Handout 5, “Naming Acids
and Bases”, in Appendix A

Unit 4 - Introduction to Acids and Bases
Curriculum Guide Chemistry 1102Page 16
Outcomes
4.2 Classify substances as acids,
bases, or salts, on the basis of their
characteristic properties.
4.2.1 Define pH scale in terms of
a measure of acidity or
alkalinity or neutrality.
4.2.2 Define acids and bases
operationally in terms of
their effect on litmus paper,
pH, sour and bitter taste,
reaction with active metals,
and reaction with each
other.
4.2.3 Define salts operationally in
terms of the conductivity of
the aqueous solutions.
4.3 Describe how neutralization
involves tempering the effects of an
acid with a base and vice versa.
Notes for Teaching and Learning
The definitions for acids and bases found in the glossary
can be used to achieve outcome 4.2.2.
Investigation 8.9, “Reacting Acids and Bases”, may be
done as an optional lab. Students should read through it
for content even if they will not complete the lab.

Unit 4 - Introduction to Acids and Bases
Curriculum Guide Chemistry 1102Page 17
Suggestions for Assessment
Questions 4.6 - 4.8 in the Study Guide should be assigned to
cover Outcomes 4.2.3 and 4.3. Students will find the answers to
these questions in Investigation 8.9 and Section 8.10 of the text.
Instructors may assign additional questions from the Chapter 8
Review.
A final examination should be given to cover the whole course.
Resources
Science 10 , Computerized
Assessment Bank.
Appendix A

Curriculum Guide Chemistry 1102Page 21
Handout 1 -“ WHMIS Activity”
1. What does WHMIS stand for?
2. What is the purpose of using WHMIS symbols?
3. What does MSDS stand for?
4. Identify the nine sections of the MSDS.
IVI
II VII
III VIII
IV IX
V
5. What is the name and chemical formula of the chemical?
6. What would happen if you were overexposed to the chemical?
7. When you are using this chemical, how would you protect yourself?
8. How must this chemical be stored?

Curriculum Guide Chemistry 1102Page 22
MSDS Sample Sheet

Curriculum Guide Chemistry 1102Page 23
Handout 2 - “Introduction to IUPAC”
Today most compounds are known by their IUPAC names. IUPAC stands for International
Union of Pure and Applied Chemistry. This organization has determined a set of rules to be
used for naming chemicals. Its purpose is to set international guidelines so that all scientists
follow the same rules.
Before you start naming compounds and writing formulas, you need to make sure you understand
the following:
Molecules are combinations of two or more elements.
*A molecular element has all atoms the same.
For example, oxygen gas is a molecule composed of 2 atoms of oxygen. It is
called a diatomic molecule (because it has 2 atoms).
Table of Diatomic Molecules
2
oxygen O
2
hydrogen H
2
nitrogen N
2
fluorine F
2
chlorine Cl
2
bromine Br
2
iodine I

Curriculum Guide Chemistry 1102Page 24
Handout 2 - “Introduction to IUPAC” ( continued )
A compound is a molecule that contains 2 or more different types of atoms or ions.
2
For example, water (H O) is a compound because it contains both hydrogen and oxygen.
2
The formula for water, H O, is a combination of symbols and subscripts.
H and O are the symbols for hydrogen and oxygen.
The number 2 is the subscript. It indicates that there are 2 atoms of hydrogen in a
molecule of water.
A molecular formula is a chemical formula that indicates the number and type of atoms in one
molecule (i.e. the actual number of atoms of each type in the compound).
An empirical formula is the simplest whole number ratio of atoms in the compound.
For example, hydrogen peroxide:
22
The molecular formula is H O
The empirical formula is HO (lowest ratio is 1:1)
Note: In some cases the molecular formula and the empirical formula are the same.

Curriculum Guide Chemistry 1102Page 25
Handout 3 - “IUPAC Naming of Compounds and Writing Formulas”
Rules for Naming Binary Ionic Compounds (simple/multivalent)
1. Name the cation (+) by writing the full name of the metal.
2. Check the attached partial periodic table to see if it is a multivalent species (has more than
one possible ionic charge).
If it has only one ionic charge, proceed to step 3.
If it has more than 1 possible ionic charge, determine the charge of the anion and pick
the metal ion that will result in a net charge of zero. Indicate the identity of the metal
ion with roman numerals.
3. Name the anion (-) by shortening the name of the atom and adding the -ide ending.
Examples: NaCl sodium chloride
2
K O potassium oxide
2
CaF calcium fluoride
4
SnCl tin(IV) chloride

Curriculum Guide Chemistry 1102Page 26
PARTIAL PERIODIC TABLE OF THE ELEMENTS
1 18
2 13 14 15 16 17
3 4 5 6 7 8 9 10 11 12
Cr
2+
Cr
3+
Mn
2+
Mn
3+
Fe
2+
Fe
3+
Co
2+
Co
3+
Cu
+
Cu
2+
Sn
2+
Sn
4+
Pb
2+
Pb
4+

Curriculum Guide Chemistry 1102Page 27
Handout 3 - “IUPAC Naming of Compounds and Writing Formulas”
Rules for Writing Formulas for Binary Ionic Compounds
1. Write the symbols of the ions involved.
2. Determine the charges of the ions.
For the cation (positive ion):
If there is no roman numeral after the name of the metal, the ion has only one ionic
charge.
If there is a roman numeral after the name of the metal, the ion has more than 1
possible ionic charge, and you must use the roman numeral to determine the charge.
For the anion (negative ion):
There is only one possible charge (recall group number).
3. Determine the lowest whole number ratio of ions that will give a net charge of zero. This
number (if something other than 1) is written as a subscript after the symbol for the ion.
4. Write the formula removing all charges.
Examples: Potassium bromide KBr
32
Calcium phosphide Ca P
2
Iron(II) chloride FeCl
Copper(I) chloride CuCl

Curriculum Guide Chemistry 1102Page 28
Handout 3 - “IUPAC Naming of Compounds and Writing Formulas”
Rules for Naming Molecular Compounds
1. Write the name of the first element in full.
2. Shorten the name of the second element and add the ide ending.
3. Use prefixes to indicate the number of atoms of each element in the molecular formula.
4. The prefix mono on the first name is optional.
Examples:
4
CCl Carbon tetrachloride
2
SiO Silicon dioxide
CO Carbon Monoxide

Curriculum Guide Chemistry 1102Page 29
Handout 3 - “IUPAC Naming of Compounds and Writing Formulas”
Rules for Writing Molecular Formulas
1. Write the symbols for each element in the compound.
2. Use the prefix to determine the number of atoms of each element in the formula and write
the appropriate number as a subscript to the right of the element’s symbol.
3. If an element lacks a prefix, assume that there is just one atom of that element. It is not
necessary to write the numerical subscript 1, since it is implied.
Examples:
26
Diboron hexahydride B H
3
Nitrogen trioxide NO

Curriculum Guide Chemistry 1102Page 30
Handout 3 - “IUPAC Naming of Compounds and Writing Formulas”
Worksheet 1

Curriculum Guide Chemistry 1102Page 31
Handout 3 - “IUPAC Naming of Compounds and Writing Formulas”
Worksheet 2

Curriculum Guide Chemistry 1102Page 32

Curriculum Guide Chemistry 1102Page 33
Worksheet 3

Curriculum Guide Chemistry 1102Page 34

Curriculum Guide Chemistry 1102Page 35
Worksheet 4

Curriculum Guide Chemistry 1102Page 36

Curriculum Guide Chemistry 1102Page 37
Worksheet 5
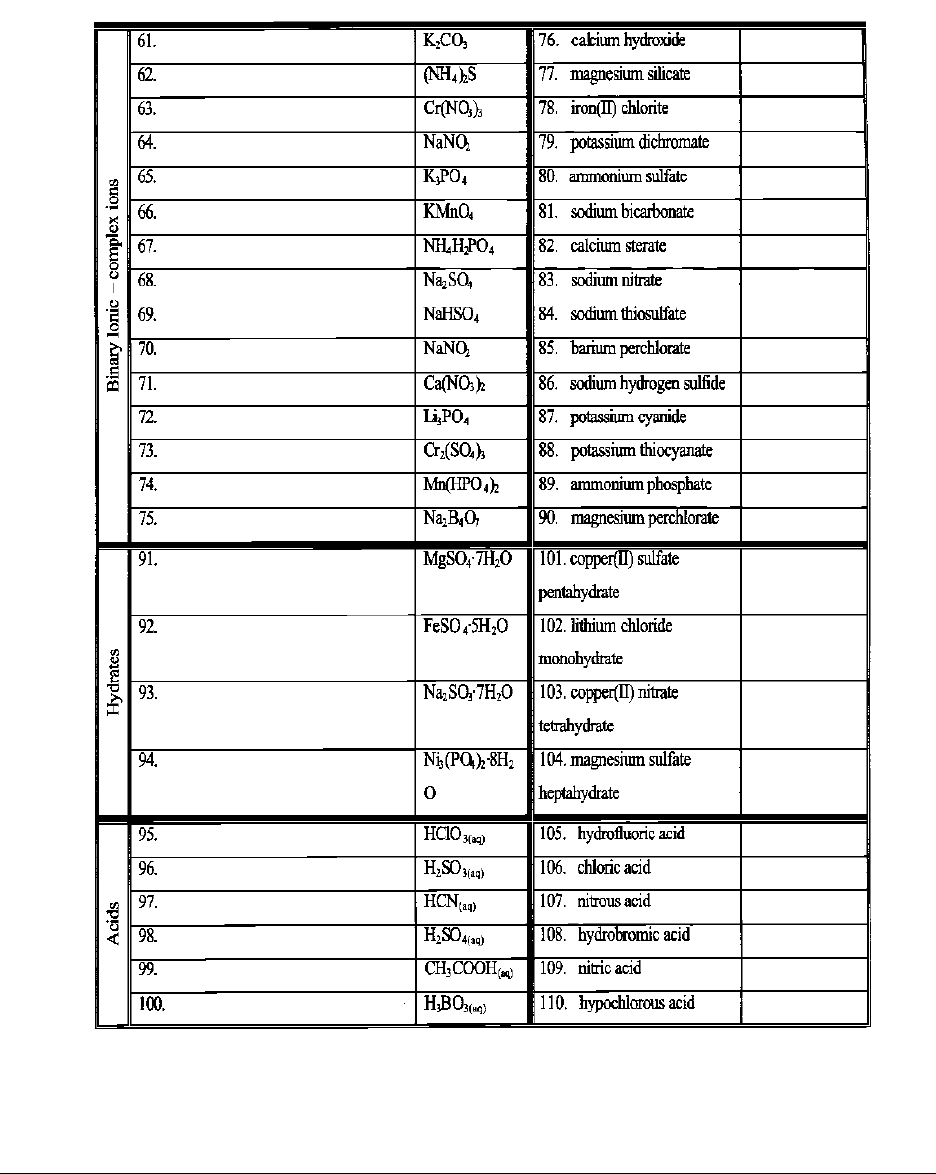
Curriculum Guide Chemistry 1102Page 38

Curriculum Guide Chemistry 1102Page 39
Handout 4 - “Naming Ionic Hydrates”
An ionic hydrate is a compound that has water associated with it. Water is part of its crystalline
structure.
The name of an ionic hydrate can be distinguished from the names of other ionic compounds by
the presence of the term hydrate with a prefix indicating the number of water molecules.
For example:
22
The IUPAC formula for calcium chloride dihydrate is CaCl @2H O.
42
The IUPAC formula for calcium magnesium sulfate heptahydrate is MgSO @7H O.
(Note the raised dot in front of the water molecules.)
In order to convert IUPAC names for ionic hydrates into chemical formulas, you will need to
know the prefixes listed below:
mono 1
di 2
tri 3
tetra 4
penta 5
hexa 6
hepta 7
octa 8
nona 9
deca 10

Curriculum Guide Chemistry 1102Page 40
Handout 4 - “Naming Ionic Hydrates”
Worksheet

Curriculum Guide Chemistry 1102Page 41
Handout 5 - “Naming Acids”
For this course, when you are given a chemical formula for a hydrogen compound that has
the (aq) state of matter subscript, you name it as an acid.
Rules for naming acids:
1. If the anion does not contain oxygen, the acid is named with the prefix hydro- and the suffix
-ic attached to the root name for the element.
(aq)
Example: HCl hydrochloric acid
(aq)
HCN hydrocyanic acid
2 (aq)
HS hydrosulfuric acid
2. If the anion contains oxygen, check the ending if the anion.
If the anion has the -ite ending, the suffix -ous is used.
Example:
3
2 3 (aq)
HSO contains the sulfite (SO ) ion and is named sulfurous acid .
2-
If the anion has the -ate endind, the suffix -ic is used..
Example:
4
2 4 (aq)
HSO contains the sulfate (SO ) ion and is named sulfuric acid .
2-

Curriculum Guide Chemistry 1102Page 42
Handout 5 - “Naming Acids”
Worksheet

Curriculum Guide Chemistry 1102Page 43

Curriculum Guide Chemistry 1102Page 44

Curriculum Guide Chemistry 1102Page 45
