
COVID-19 IN
NURSING HOMES
CMS Needs to
Continue to
Strengthen Oversight
of Infection Prevention
and Control
Report to Congressional Addressees
September 2022
GAO-22-105133
United States Government Accountability Office

United States Government Accountability Office
Highlights of GAO-22-105133, a report to
congressional a
ddressees
September 2022
COVID-19 IN NURSING HOMES
CMS
Needs to Continue to Strengthen Oversight of
Infection
Prevention and Control
What GAO Found
The Centers for Medicare & Medicaid Services (CMS) is responsible for ensuring
that nursing homes meet federal standards. CMS enters into agreements with
state survey agencies to conduct surveys and investigations of the state’s
nursing homes. The Centers for Disease Control and Prevention (CDC) issues
guidance, operates surveillance systems, and provides technical assistance to
support infection prevention and control in nursing homes.
GAO analysis of CMS data reported by nursing homes shows that seven of the
eight key indicators of nursing home resident mental and physical health
worsened at least slightly the first year of the pandemic (2020), compared to the
years prior to the pandemic. See the figure below for examples of two outcomes
we reviewed.
Percentage of Residents Who Experienced Depression and Unexplained Weight Loss, by Year
CMS and CDC took actions on infection prevention and control prior to and
during the COVID-19 pandemic. For example, prior to the pandemic, CMS
required nursing homes to designate an infection preventionist on staff. This
person is a trained employee responsible for the home’s infection prevention and
control program and was crucial to nursing homes during the pandemic. CMS
also made changes in how nursing homes were surveyed during the pandemic.
However, GAO found areas where CMS could take additional actions, including:
• Strengthening oversight of the infection preventionist role. GAO identified
ways CMS could strengthen oversight of the infection preventionist role, such
as by establishing minimum training standards. CMS could also collect
infection preventionist staffing data and use it to determine whether the current
infection preventionist staffing requirement is sufficient.
• Strengthening infection prevention and control guidance. GAO identified
how CMS could strengthen this guidance by providing information to help
surveyors assess the scope and severity of infection prevention and control
deficiencies they identify. For example, CMS could add COVID-19-relevant
examples for scope and severity classifications to its State Operations
Manual—the key guidance state survey agencies use for conducting nursing
home surveys.
View GAO-22-105133. For more information,
contact
John Dicken at (202) 512-7114 or
.
Why GAO Did This Study
Implementing proper infection
prevention and
control practices can
be critical for preventing the spread of
infectious diseases.
Infection
prevention and control
has been a
long
-standing concern in the nation’s
more than 15,000 nursing homes
—
one
that the COVID
-19 pandemic has
brought into sharper focus.
Some
infection prevention and control
practices in nursing h
omes, such as
social isolation,
may negatively affect
resident mental and physical health.
The CARES Act
directs GAO to
monitor the federal pandemic
response. GAO was also asked to
review
federal oversight of nursing
homes in light of the pandemic. Among
other objectives, this report: (1)
describes
what data reveal about any
changes in resident health
before and
during the pandemic and (2)
examines
infection prevention and control actions
CMS and CDC have
taken in nursing
homes before and
during the
pandemic.
GAO
(1) reviewed CMS and CDC
documents
, (2) analyzed CMS
resident
health
data from 2018 through 2021,
and
(3) interviewed CMS, CDC, state
survey agency,
and nursing home
officials
in a non-generalizable sample
of eight
states selected for variation in
factors such as geographic location.
What GAO
Recommends
GAO is making
three
recommendations to
CMS related to
the role of the infection preventionist
and
clarifying infection prevention and
control
guidance. HHS agree
d with our
first recommendation
, but neither
agreed nor disagreed with our other
two
recommendations.

Page i GAO-22-105133 Nursing Home Infection Control
Letter 1
Background 6
Some Indicators of Resident Mental and Physical Health
Worsened during the COVID-19 Pandemic 10
Infection Prevention and Control Deficiencies Persisted in Nursing
Homes during the COVID-19 Pandemic 15
CMS and CDC Took Actions to Strengthen Infection Prevention
and Control but Should Do More 21
Conclusions 33
Recommendations for Executive Action 33
Agency Comments and Our Evaluation 34
Appendix I Related GAO Products on COVID-19 in Nursing Homes 38
Appendix II Examples of Infection Prevention and Control Deficiencies Cited
in Nursing Homes during the Pandemic 39
Appendix III Types of Surveys and Investigations to Assess Whether
Nursing Homes Are Meeting Federal Standards 40
Appendix IV Number and Percentage of Surveyed Nursing Homes with Infection
Prevention and Control (IPC) Deficiencies 44
Appendix V Federal Nursing Home Infection Prevention and Control (IPC) Actions 45
Appendix VI Comments from the Department of Health and Human Services 49
Appendix VII GAO Contact and Staff Acknowledgments 55
Contents

Page ii GAO-22-105133 Nursing Home Infection Control
Tables
Table 1: Selected Federal Infection Prevention and Control (IPC)
Actions and Examples of Stakeholder-Reported
Perspectives on Advantages and Disadvantages 26
Table 2: Illustrative Examples of Narratives from Infection
Prevention and Control Deficiencies Cited in Nursing
Homes during the Pandemic 39
Table 3: Number and Percentage of Surveyed Nursing Homes
with Infection Prevention and Control (IPC) Deficiencies,
by Calendar Year and Deficiency Code 44
Table 4: Nursing Home Infection Prevention and Control (IPC)
Actions Taken by the Centers for Medicare & Medicaid
Services (CMS) and the Centers for Disease Control and
Prevention (CDC) 45
Figures
Figure 1: Percentage of Long-Stay Nursing Home Residents Who
Experienced Selected Mental Health Indicators, by Year 11
Figure 2: Percentage of Long-Stay Nursing Home Residents Who
Experienced Selected Physical Health Indicators, by Year 12
Figure 3: Type of Survey or Investigation Used by State Survey
Agencies to Identify Infection Prevention and Control
Deficiencies, 2018 through 2021 17
Figure 4: Types of Surveys and Investigations Used by State
Survey Agencies to Assess Whether Nursing Homes Are
Meeting Federal Standards, as of April 2022 42

Page iii GAO-22-105133 Nursing Home Infection Control
Abbreviations
CDC Centers for Disease Control and Prevention
CMS Centers for Medicare & Medicaid Services
HHS Department of Health and Human Services
IPC Infection Prevention and Control
This is a work of the U.S. government and is not subject to copyright protection in the
United States. The published product may be reproduced and distributed in its entirety
without further permission from GAO. However, because this work may contain
copyrighted images or other material, permission from the copyright holder may be
necessary if you wish to reproduce this material separately.

Page 1 GAO-22-105133 Nursing Home Infection Control
441 G St. N.W.
Washington, DC 20548
September 14, 2022
Congressional Addressees
Infection prevention and control (IPC) has been a long-standing concern
in the nation’s more than 15,000 Medicare- and Medicaid-certified nursing
homes—one the COVID-19 pandemic has brought into sharper focus.
1
Prior to the COVID-19 pandemic, infections were a leading cause of
death and hospitalization among nursing home residents, with estimates
of up to 380,000 residents dying each year.
2
Since that time, COVID-19
has emerged as a new and highly contagious respiratory disease that has
had devastating consequences for the nation’s more than one million
nursing home residents, including high rates of severe illness and death.
COVID-19 has also substantially affected the broader nursing home
industry, including nursing home staff. The initial unknown nature of the
virus that causes COVID-19 and the scope of the pandemic also created
unprecedented challenges for state and federal agencies that work to
ensure the quality of care delivered in nursing homes and to protect
public health.
3
In our previous reporting, we found that, in the years prior to the
pandemic, nursing homes had persistent and widespread challenges with
IPC.
4
For example, we found that implementing proper IPC practices,
such as isolating infected residents, can be critical for preventing the
spread of infectious diseases, including COVID-19—thus protecting both
resident and staff health and well-being. However, some IPC practices in
1
According to the Centers for Disease Control and Prevention, IPC protects patients,
residents, healthcare personnel, and visitors by preventing healthcare-associated
infections and limiting the spread of pathogens through the implementation of evidence-
based interventions.
2
Department of Health and Human Services, The National Action Plan to Prevent Health
Care-Associated Infections: Road Map to Elimination (Washington, D.C.: 2013).
3
As GAO has previously reported, during the COVID-19 pandemic, nursing homes
experienced high staff cases and deaths and challenges related to staffing, personal
protective equipment, and testing. See, for example, GAO, COVID-19: Federal Efforts
Could Be Strengthened by Timely and Concerted Actions, GAO-20-701 (Washington,
D.C.: Sept. 21, 2020).
4
GAO, Infection Control Deficiencies Were Widespread and Persistent in Nursing Homes
Prior to COVID-19 Pandemic, GAO-20-576R (Washington, D.C.: May 20, 2020).
Letter

Page 2 GAO-22-105133 Nursing Home Infection Control
nursing homes, such as social isolation, may negatively affect resident
mental and physical health.
5
The Department of Health and Human Services (HHS), primarily through
the Centers for Medicare & Medicaid Services (CMS) and the Centers for
Disease Control and Prevention (CDC), has led the response to the
COVID-19 pandemic in nursing homes. CMS is the federal oversight
agency responsible for ensuring that nursing homes meet federal quality
standards to be eligible to participate in the Medicare and Medicaid
programs. These standards require, for example, that nursing homes
establish and maintain an IPC program. To monitor compliance with
these standards, CMS enters into agreements with state survey agencies
in each state government and oversees the work the state survey
agencies do. CDC issues guidance with recommendations for preventing
and managing infectious diseases, operates infectious disease
surveillance systems, and provides technical assistance through
programs aimed at supporting and assessing IPC in nursing homes, and
tracking IPC data.
The CARES Act includes a provision directing us to monitor the federal
response to the COVID-19 pandemic.
6
Further, you also asked us to
examine federal oversight of IPC protocols and the adequacy of
emergency preparedness standards for emerging infectious diseases in
nursing homes, as well as CMS’s response to the pandemic. Since 2020,
we have examined the response to COVID-19 in nursing homes in
multiple studies. Some studies have been completed and released and
others are ongoing. (See app. I for a list of completed related reports.)
In this report, we: (1) describe what data reveal about any changes in
resident mental and physical health before and during the COVID-19
pandemic, (2) describe whether IPC challenges have persisted in nursing
homes during the pandemic, and (3) examine the IPC actions that CMS
5
National Academies of Sciences, Engineering, and Medicine, Social Isolation and
Loneliness in Older Adults: Opportunities for the Health Care System (Washington, D.C.:
The National Academies Press, 2020).
6
Pub. L. No. 116-136, § 19010(b), 134 Stat. 281, 580 (2020). Throughout the pandemic,
we regularly issued government-wide reports on the federal response to COVID-19. All
government-wide reports are available on GAO’s website at
https://www.gao.gov/coronavirus.

Page 3 GAO-22-105133 Nursing Home Infection Control
and CDC have taken related to nursing homes before and during the
pandemic.
To describe what data reveal about any changes in resident mental and
physical health before and during the COVID-19 pandemic, we analyzed
2018 through 2021 CMS Minimum Data Set resident assessment data.
7
We compared selected health indicators across calendar years for all
long-stay residents who had lived in the nursing home greater than 100
days.
8
We selected four mental and four physical health indicators to
analyze based on indicators highlighted in our review of relevant literature
and during conversations with knowledgeable stakeholders.
9
Then, using
each resident’s calendar year assessments, we determined the
percentage of residents experiencing each selected health indicator.
10
We
analyzed the data in the CMS Minimum Data Set as they were reported
by nursing homes to CMS. We did not otherwise independently verify the
accuracy of the information with these nursing homes. We assessed the
reliability of the dataset by checking for missing values and obvious
errors, reviewing relevant CMS documents, and reviewing other studies
7
2021 was the most recent calendar year available at the time of our analysis.
The CMS Minimum Data Set is reported by nursing homes, which are required to
complete resident assessments at regular intervals as part of federal requirements to
participate in the Medicare and Medicaid programs. Nursing homes are required to
conduct resident assessments at entry, quarterly, at discharge, and if there are any
significant changes or corrections. During standard surveys, surveyors can evaluate
whether a nursing home’s assessments meet federal standards for accuracy.
8
The same resident may have lived in the home for multiple years and would therefore be
present in each calendar year. Most nursing homes provide both long-term residential and
short-term rehabilitative care.
According to CMS, the number of nursing home residents declined sharply during the
pandemic.
9
For example, see M. Levere, P. Rowan, A. Wysocki, “The Adverse Effects of the COVID-
19 Pandemic on Nursing Home Resident Well-Being,” JAMDA, vol. 22, no. 5 (2021): 948-
954 and L. Fleisher et al., “Health Care Safety During the Pandemic and Beyond—
Building a System That Ensures Resilience,” The New England Journal of Medicine, vol.
386, no. 7 (2022): 609-611.
10
The mental health indicators we selected and analyzed included whether, on any
assessment in a given calendar year, a resident had any symptoms of depression or took
anti-depressant, anti-psychotic, or anti-anxiety medications. The physical health indicators
we selected and analyzed included whether, on any assessment in a given calendar year,
a resident experienced at least one fall, unexplained weight loss, urinary incontinence
ranging from occasionally incontinent to always incontinent, or at least one or more stage
1 or higher unhealed pressure ulcers.

Page 4 GAO-22-105133 Nursing Home Infection Control
that used these data and identified some limitations of our analysis.
11
Based on this review, we determined the data were sufficiently reliable for
the purposes of this reporting objective. We also conducted interviews
with officials from a non-generalizable sample of nine selected nursing
homes in eight selected states: Arkansas, California, Florida, Maryland,
Michigan, Montana, Rhode Island, and Washington.
12
These states were
selected based on three criteria: (1) geographic location; (2) number of
nursing home beds; and (3) number of nursing home residents and staff
with confirmed positive cases of COVID-19.
13
We then selected nursing
homes to obtain variation in factors such as bed count and profit or not-
for-profit status. We asked nursing home officials to describe resident
mental and physical health during the pandemic. Additionally, we
interviewed national associations, including the American Health Care
Association and National Consumer Voice for Quality Long-Term Care,
about these issues.
To describe whether IPC challenges have persisted in nursing homes
during the pandemic, we analyzed CMS data on nursing home
deficiencies cited by state surveyors in all 50 states and Washington,
11
Some studies have found that the Minimum Data Set data reported by nursing homes
underreports anti-psychotic use and falls. Therefore, it is possible that our analysis also
underreports these health indicators. For examples, see HHS Office of Inspector General,
CMS Could Improve the Data It Uses to Monitor Antipsychotic Drugs in Nursing Homes,
OEI-07-19-00490 (Washington, D.C.: May 3, 2021) and J. Mintz et al., “Validation of the
Minimum Data Set Items on Falls and Injury in Two Long-Stay Facilities,” Journal of the
American Geriatrics Society, vol. 69, no. 4 (April 2021). Unless the rate of underreporting
changed during the pandemic, the analysis of change over time would still likely be
broadly valid.
In addition, as the pandemic progressed, it is possible that nursing homes had to delay
submitting their resident assessments if, for example, they were responding to a COVID-
19 outbreak. In March 2020, CMS waived the timeframe requirements for nursing homes
to complete and transmit resident assessments in order to allow nursing homes to focus
on infection control efforts. However, these timeframes were re-instated by CMS in April
2021. According to CMS, the majority of nursing homes were completing and transmitting
their assessments in a timely fashion. This is consistent with our analysis, where we
determined that less than 10 percent of nursing home quarterly assessments were
delayed in each year of our review. See Centers for Medicare & Medicaid Services,
Updates to Long-Term Care Emergency Regulatory Waivers Issued in Response to
COVID-19, QSO-21-17-NH (Baltimore, Md.: April 8, 2021).
12
In Washington State, we interviewed officials from two nursing homes, while in the other
states, we interviewed officials from one home in each state.
13
COVID-19 case rates were for the week ending May 16, 2021.

Page 5 GAO-22-105133 Nursing Home Infection Control
D.C., from 2018 through 2021.
14
Using these data, we analyzed the
deficiency codes used by state surveyors when a nursing home fails to
meet CMS’s requirements for IPC. We also used CMS’s Quality,
Certification, and Oversight Reports website to obtain high-level summary
data on the percentage of nursing homes with an overdue standard
survey.
15
We assessed the reliability of these datasets by checking for
missing values and obvious errors and reviewing relevant CMS
documents and determined the data were sufficiently reliable for the
purposes of this reporting objective. We also conducted interviews with
state survey agency officials and nursing home officials in the non-
generalizable sample of eight states described above. We asked
interviewees to describe the extent to which IPC challenges persisted in
nursing homes and how they have responded.
To examine the IPC actions that CMS and CDC have taken related to
nursing homes before and during the pandemic, we reviewed CMS and
CDC regulations and guidance. We also interviewed officials at CMS and
CDC and officials from state survey agencies and nine nursing homes in
the non-generalizable sample of eight states described above, as well as
officials from the national associations with knowledge of nursing home
issues previously noted. We determined that the control environment
component of internal control was significant to this objective, along with
the underlying principle that management should establish expectations
of competence for key roles. We also determined that the risk
assessment component of internal control was significant to this
objective, along with the underlying principle that management should
define objectives clearly to enable the identification of risks and define
risk tolerances. Finally, we determined that the information and
communication component of internal control was significant to this
objective, along with the underlying principle that management should
use quality information to achieve the entity’s objectives. We assessed
CMS’s oversight activities implemented leading up to and during the
COVID-19 pandemic in the context of these internal control principles, as
well as HHS statutory requirements, CMS regulatory requirements for
14
2021 was the most recent calendar year available at the time of our analysis.
In addition, we used the CMS Care Compare Inspection Date files, which were accessed
on March 28, 2022 from https://data.cms.gov/provider-data/dataset/svdt-c123.
15
CMS’s Quality, Certification, and Oversight Reports system is a website that provides
summary-level data reports on nursing homes. This system is available at
https://qcor.cms.gov and was accessed on April 7, 2022.

Page 6 GAO-22-105133 Nursing Home Infection Control
nursing home participation in Medicare and Medicaid programs, and
CMS’s State Operations Manual, to determine whether these oversight
actions were clearly defined and understood to enable nursing homes
and state survey agencies to address the risk posed by COVID-19; and
whether the agency has access to quality information about whether its
oversight actions were achieving their stated objectives.
16
We conducted this performance audit from April 2021 to September 2022
in accordance with generally accepted government auditing standards.
Those standards require that we plan and perform the audit to obtain
sufficient, appropriate evidence to provide a reasonable basis for our
findings and conclusions based on our audit objectives. We believe that
the evidence obtained provides a reasonable basis for our findings and
conclusions based on our audit objectives.
Federal law requires nursing homes to keep residents safe from
infectious diseases by establishing and maintaining an IPC program
designed to help prevent the development and transmission of
communicable diseases and infections.
17
Even before COVID-19, nursing home residents were at a high risk for
several different types of infections, including respiratory infections,
gastroenteritis, skin and soft tissue infections, and urinary tract infections.
Nursing home residents can be particularly susceptible to infections
because of their advanced age and higher risk of comorbidities.
18
Further,
nursing home residents are increasingly requiring more medically
complex care and are therefore more susceptible to infection. For
example, residents discharged from the hospital back to the nursing
16
Federal law establishes minimum requirements nursing homes must meet to participate
in the Medicare and Medicaid programs, and designates the HHS Secretary as
responsible to ensure that requirements governing the provision of care in nursing homes,
and the enforcement of such requirements, are adequate to protect the health, safety,
welfare, and rights of residents and promote the effective and efficient use of public
moneys. 42 U.S.C. §§ 1395i-3(f)(1),1396r(f)(1); 42 C.F.R. §§ 483.1--483.95 (2021).
GAO, Standards for Internal Control in the Federal Government, GAO-14-704G
(Washington, D.C.: Sept. 10, 2014). Internal control is a process effected by an entity’s
oversight body, management, and other personnel that provides reasonable assurance
that the objectives of an entity will be achieved.
17
42 U.S.C. §§ 1395i-3(d)(3)(A), 1396r(d)(3)(A); 42 C.F.R. § 483.80 (2021).
18
Comorbidity refers to the presence of more than one distinct disease in a person at the
same time.
Background
Infections in Nursing
Homes

Page 7 GAO-22-105133 Nursing Home Infection Control
home can bring infections into the home. They may also require a high-
degree of clinical monitoring in order to identify and prevent infection and
to help prevent the spread of resistant pathogens between residents. In
addition, while nursing homes create important social opportunities for
residents through communal dining and recreational spaces, these
shared spaces can increase the transmission risk for infectious diseases,
especially viruses causing respiratory or gastrointestinal outbreaks.
The COVID-19 pandemic has led to high rates of infection and death in
nursing home residents and staff. Nursing home residents are at
increased risk because older adults and those with underlying health
conditions have a high mortality rate when infected with the virus,
according to CDC.
19
In addition, the congregate nature of nursing
homes—with staff caring for multiple residents and residents sharing
rooms and other communal spaces—can increase the risk that COVID-19
will enter the home and easily spread.
20
The introduction of COVID-19
vaccines in December 2020 resulted in a sharp decline in nursing home
cases and deaths through the first part of 2021; however, cases and
deaths began to increase again with the emergence of more highly
transmissible virus variants during the summer of 2021, coinciding with
the emergence of the Delta variant, and again in winter 2022, coinciding
with the emergence of the Omicron variant.
Federal laws establish minimum requirements nursing homes must meet
to participate in the Medicare and Medicaid programs, including
standards for the quality of care.
21
Primarily through its State Operations
Manual, CMS establishes the responsibilities of state survey agencies in
ensuring that these federal quality standards for nursing homes are met,
19
Centers for Disease Control and Prevention, COVID-19: People Who Live in a Nursing
Home or Long-Term Care Facility, accessed on May 7, 2022,
https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-in-nursing-ho
mes.html.
20
According to CDC, COVID-19 is spread in three main ways: (1) breathing in small
droplets or particles exhaled by an infected person (2) having these small droplets and
particles land on the eyes, nose, or mouth, especially through a cough or a sneeze (3)
touching eyes, nose, or mouth with hands that have the virus on them. See Centers for
Disease Control and Prevention, How COVID-19 Spreads, accessed on April 16, 2022,
https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html.
21
42 U.S.C. §§ 1395i-3, 1396r; 42 C.F.R. §§ 483.1--483.95 (2021). Federal statutes and
their implementing regulations use the terms “skilled nursing facility” (Medicare) and
“nursing facility” (Medicaid). For the purposes of this report, we use the term nursing home
to refer to both skilled nursing facilities and nursing facilities.
Federal Oversight of
Nursing Homes

Page 8 GAO-22-105133 Nursing Home Infection Control
such as that nursing homes establish and maintain an IPC program.
22
To
monitor compliance with these standards, CMS enters into agreements
with state survey agencies in each state to assess whether nursing
homes meet CMS’s standards. Prior to the pandemic, state surveyors
from the state survey agencies were responsible for assessing nursing
homes using (1) recurring comprehensive standard surveys, or (2) as-
needed investigations for complaints from the public and facility-reported
incidents.
• Standard surveys. State survey agencies are required by federal law
to perform unannounced, on-site standard surveys of every nursing
home receiving Medicare or Medicaid payment at least every 15
months, with a statewide average frequency of every 12 months.
23
Standard surveys are important for protecting nursing home residents
because they serve as a comprehensive assessment of the safety
and quality of nursing home care across several areas including food
and nutrition, resident rights, physician and nursing services, and the
physical environment.
• Investigations. In addition to performing standard surveys, state
survey agencies are required by federal law to investigate all
complaints of nursing home violations of requirements.
24
These fall
into two categories: (1) complaints submitted by residents, family
members, friends, physicians, and nursing home staff; and (2)
“facility-reported incidents” that are self-reported by the nursing
homes. These investigations offer the state survey agency a unique
opportunity to identify and correct care problems, as they can provide
a timely alert of acute issues that otherwise might not be addressed
until a standard survey takes place.
If a surveyor from a state survey agency determines that a nursing home
violated a federal standard during a survey or investigation, the nursing
22
At a minimum, nursing homes must (1) have a system to prevent, identify, report,
investigate, and control infections and communicable diseases for all residents, staff,
volunteers, visitors, and others providing services in the home; (2) have written standards,
policies, and procedures for their infection prevention and control program; (3) have
antibiotic use protocols and a system to monitor antibiotic use; and (4) have a system for
recording incidents identified under the home’s infection prevention and control program
and any corrective actions taken. 42 C.F.R. § 483.80(a)(1)-(4) (2021).
23
42 U.S.C. §§ 1395i-3(g)(1)(A), (g)(2)(A)(iii), 1396r(g)(1)(A), (g)(2)(A)(iii); 42 C.F.R. §
488.308(a)-(b) (2021).
24
42 U.S.C. §§ 1395i-3(g)(4), 1396r(g)(4); 42 C.F.R. § 488.332(a) (2021).

Page 9 GAO-22-105133 Nursing Home Infection Control
home is cited for the deficiency using a specific deficiency code (referred
to as an F-tag). Cited deficiencies are then classified into categories
according to scope (the number of residents potentially affected) and
severity (the potential for or occurrence of harm to residents). For most
cited deficiencies, nursing homes are required to submit a plan of
correction that addresses how the home plans to correct the
noncompliance and implement systemic change to ensure the deficient
practice would not recur.
25
In addition, when nursing homes are cited with
deficiencies, federal enforcement actions can be implemented to
encourage homes to make corrections.
26
In general, for deficiencies with
a higher scope and severity, CMS may implement the enforcement action
immediately.
27
For other deficiencies with a lower scope and severity, the
nursing home may be given an opportunity to correct the deficiencies,
which, if corrected before the scheduled effective date, can result in the
planned enforcement action not being implemented.
In 2016, CMS finalized a comprehensive update to its nursing home
standards.
28
The update included new requirements and aligned existing
requirements with current clinical practices. These standards covered a
variety of categories, such as quality of care and IPC.
We have issued several reports examining COVID-19 in nursing homes,
part of our larger bodies of work on nursing home oversight and on the
federal response to the COVID-19 pandemic (see app. I.) For example, in
May 2020, we analyzed CMS deficiency data and found that most nursing
25
The plan of correction serves as the nursing home’s allegation of compliance.
Depending on the severity of the deficiency cited, surveyors revisit the nursing home to
ensure that the home actually implemented its plan and corrected the deficiency.
26
CMS does not require enforcement actions be implemented for all deficiencies.
Enforcement actions include, but are not limited to, directed in-service training, fines
known as civil money penalties, denial of payment, and termination from the Medicare and
Medicaid programs.
27
The scope and severity of a deficiency is one of the factors that CMS may take into
account when implementing enforcement actions. CMS may also consider a nursing
home’s prior compliance history, desired corrective action and long-term compliance, and
the number and severity of all the nursing home’s deficiencies.
28
Medicare and Medicaid Programs; Reform of Requirements for Long-Term Care
Facilities, 81 Fed. Reg. 68,688 (Oct. 4, 2016). Phase 1 (effective November 28, 2016)
implemented most minor modifications to the existing nursing home regulations; phase 2
(effective November 28, 2017) implemented new regulations and re-structured CMS’s
deficiency code system; and phase 3 (effective November 28, 2019) implemented the
remaining requirements.
Prior GAO Work

Page 10 GAO-22-105133 Nursing Home Infection Control
homes were cited for IPC deficiencies, such as failure to use proper hand
hygiene, in the years prior to the COVID-19 pandemic.
29
In addition,
during the COVID-19 pandemic, most nursing homes had multiple
outbreaks and weeks of sustained COVID-19 transmission from May
2020 through January 2021.
30
In response to the CARES Act, we have
examined the federal response to COVID-19 in nursing homes in multiple
reports, where we reported on nursing home-related actions HHS had
taken in response to the pandemic, as well as challenges nursing homes
faced responding to COVID-19.
Our analysis of CMS data shows that seven of the eight key indicators of
nursing home resident mental and physical health that we reviewed
worsened at least slightly in 2020, the first year of the pandemic,
compared to the years prior to the pandemic.
31
Six of these key indicators
continued to be worse in the second year of the pandemic than in the
years prior to the pandemic.
32
For example, the percentage of residents
who experienced depression was 58.7 percent in 2018, 63.9 percent in
2020, and 61.5 percent in 2021. Similarly, the percentage of residents
who experienced unexplained weight loss was 14.8 percent in 2018, 19.3
percent in 2020, and 17.4 percent in 2021. (See figures 1 and 2.)
29
See GAO-20-576R.
30
GAO, COVID-19 in Nursing Homes: Most Homes Had Multiple Outbreaks and Weeks of
Sustained Transmission from May 2020 through January 2021, GAO-21-367
(Washington, D.C.: May 19, 2021).
31
During the COVID-19 pandemic, there have been concerns about mental health in the
general population. For example, in January 2021, four in 10 U.S. adults reported
symptoms of anxiety or depressive disorder, up from one in 10 in 2019. See N. Panchal,
R. Kamal, C. Cox, and R. Garfield, The Implications of COVID-19 for Mental Health and
Substance Abuse (San Francisco, Calif.: Henry J. Kaiser Family Foundation, 2021).
32
We observed a large decrease (44 percent) in the number of long-stay nursing home
residents between 2018 and 2021 (from about 1.9 million to 1.0 million). CMS officials
indicated that they also observed a sharp decline in the number of nursing home residents
during the pandemic. It is likely that more residents left nursing homes or passed away
during the pandemic, either due to COVID-19 or other factors, compared to prior years. It
is unclear whether the residents who remained in nursing homes during the pandemic in
2020 and 2021 had different health issues than residents who lived in nursing homes prior
to the pandemic.
Some Indicators of
Resident Mental and
Physical Health
Worsened during the
COVID-19 Pandemic

Page 11 GAO-22-105133 Nursing Home Infection Control
Figure 1: Percentage of Long-Stay Nursing Home Residents Who Experienced
Selected Mental Health Indicators, by Year
Notes: Long-stay residents are those living in a nursing home for greater than 100 days. The data in
the Minimum Data Set are self-reported to CMS by nursing homes. “Experienced depression”
indicates whether a resident had any symptoms of depression on any assessment in a given calendar
year. “Took anti-depressant medications,” “took anti-psychotic medications,” and “took anti-anxiety
medications” indicates if, on any assessment in a given calendar year, a resident took anti-
depressant, anti-psychotic, or anti-anxiety medications in the prior 7 days before the assessment or, if
less than 7 days, since admission.

Page 12 GAO-22-105133 Nursing Home Infection Control
Figure 2: Percentage of Long-Stay Nursing Home Residents Who Experienced
Selected Physical Health Indicators, by Year
Notes: Long-stay residents are those living in a nursing home for greater than 100 days. The data in
the Minimum Data Set are self-reported to CMS by nursing homes. “Experienced at least one fall”
indicates if, on any assessment in a given calendar year, a resident experienced at least one fall
since the prior assessment or since admission, whichever was more recent. “Experienced
unexplained weight loss” indicates if, on any assessment in a given calendar year, a resident
experienced weight loss of 5 percent or more in the last month or 10 percent or more in the last six
months. “Experienced incontinence” indicates if, on any assessment in a given calendar year, a
resident experienced urinary incontinence ranging from occasionally incontinent to always
incontinent. “Experienced at least one pressure ulcer” indicates if, on any assessment in a given
calendar year, a resident had at least one or more stage 1 or higher unhealed pressure ulcers.

Page 13 GAO-22-105133 Nursing Home Infection Control
The results of our data analysis were supported by our interviews with
nursing home officials in selected states, who told us they observed
worsening resident mental and physical health during the COVID-19
pandemic. Specifically, for resident mental health, officials from some
nursing homes we interviewed told us they observed more residents who
experienced depression, as well as more residents who took anti-
psychotic medication. Nursing home officials and national organizations
we interviewed attributed this in part to the isolation residents felt from the
limitations CMS placed on visitation or group activities in nursing homes
during the pandemic to limit the transmission of COVID-19. CMS initially
restricted visitation and suspended group activities in March 2020. After
the initial restrictions, CMS made changes to its guidance multiple times
during the pandemic to allow for more visitation and group activities, while
identifying some situations where limitations would be appropriate to help
prevent COVID-19 infections. In November 2021, all visitation limitations
were fully lifted.
33
According to CDC, these restrictions were intended to
help limit transmission of COVID-19 early in the pandemic, when nursing
homes faced multiple complex challenges, including: understanding a
novel virus, inability to test to detect asymptomatic infected individuals,
variable personal protective equipment supply access, staffing shortages
that made controlled visitation more difficult, increasing cases across the
country with few effective treatments available, and no vaccine
availability.
Nursing home officials in our selected states also told us that they
observed worsening resident physical health during the COVID-19
pandemic. Officials from some of the nursing homes we interviewed told
us they observed more residents who experienced weight loss and falls
when visitation and group activities were limited. One factor contributing
to unintended weight loss by residents may have been that, prior to the
pandemic, visitors assisted some residents with eating. Officials from one
nursing home said that these residents did not eat as well when being fed
by a busy staff member rather than an attentive visitor and thus lost
33
These restrictions began in March 2020, were changed in September 2020, March
2021, and April 2021, and were fully lifted for all residents in November 2021. See Centers
for Medicare & Medicaid Services, Guidance for Infection Control and Prevention of
COVID-19 in Nursing Homes, QSO-20-14-NH (Baltimore, Md.: March 13, 2020) and
Centers for Medicare & Medicaid Services, Nursing Home Visitation – COVID-19, QSO-
20-39-NH (Baltimore, Md.: Sept. 17, 2020), revised March 10, 2021, April 27, 2021, and
November 12, 2021. CMS also released question and answer documents to help enable
visitation and frequently asked question documents on visitation, such as this example
published March 10, 2022:
https://www.cms.gov/files/document/nursing-home-visitation-faq-1223.pdf accessed June
8, 2022.

Page 14 GAO-22-105133 Nursing Home Infection Control
weight. Officials from another nursing home said that residents were at a
higher risk for falls for various reasons including, for example, they were
alone in their rooms and would try to move independently without staff
assistance or with inadequate staff assistance. According to CMS, some
nursing homes may have been overly restrictive on visitation in a manner
that was inconsistent with CMS guidance. CMS noted that the agency
requires nursing homes to implement care plans for each resident to
attain or maintain the resident’s highest practicable physical, mental, and
psychosocial well-being.
In November 2021, CMS updated its guidance to allow visitation and
group activities with no restrictions, noting that the agency recognized
that physical separation from family had taken a physical and emotional
toll on residents.
34
Officials from some of the nursing homes we
interviewed described seeing a visible improvement in residents once
visitation and group activities were allowed again. For example, officials
from one selected nursing home said that depression decreased and
residents began eating better.
There may be other factors that have contributed to worsening resident
mental and physical health during the pandemic. For example, in April
2022, CMS cited significant concerns with the quality of resident care
identified by surveyors, such as weight loss, depression, and pressure
ulcers as a key rationale for its plans to end certain emergency blanket
waivers issued during the pandemic, such as waived training
requirements for certified nurse aides.
35
In addition, according to one
study we reviewed, changes in nursing home resident well-being could be
the result of a variety of causes, including the direct effects of being sick
with COVID-19, fears associated with contracting the virus, grief from
losing friends and loved ones, changes in care practices, such as the
34
See Centers for Medicare & Medicaid Services, QSO-20-39-NH (November 12, 2021
revision).
35
CMS noted that by waiving these training requirements, certified nurse aides may not
have received the necessary training to, for example, help identify and prevent weight loss
in residents. As a result, CMS stated that the agency is concerned about how residents’
health and safety has been impacted by the regulations that have been waived. See
Centers for Medicare & Medicaid Services, Update to COVID-19 Emergency Declaration
Blanket Waivers for Specific Providers, QSO-22-15-NH (Baltimore, Md.: April 7, 2022).

Page 15 GAO-22-105133 Nursing Home Infection Control
declines in the provision of therapy, and other policies put in place to limit
the spread of the virus.
36
The percentage of nursing homes cited for infection prevention and
control deficiencies during the COVID-19 pandemic was generally
consistent with the years prior. Nursing homes received IPC deficiencies
during the COVID-19 pandemic in 2020 and 2021 for failing to follow
basic practices, such as proper handwashing, but also for failing to follow
COVID-19-specific practices. Officials from the state survey agencies we
interviewed said the most persistent IPC challenges in nursing homes
during the pandemic were often attributed to staffing challenges. Despite
these challenges, stakeholders we interviewed said that nursing homes
had gained valuable knowledge about IPC during the pandemic.
Our analysis of CMS data shows that the percentage of nursing homes
cited for infection prevention and control deficiencies in 2020 and 2021
was generally consistent with the years prior.
37
Specifically, about 44
percent of nursing homes were cited for at least one IPC deficiency in
2020, which decreased to about 37 percent in 2021. Prior to the
pandemic, in 2018 and 2019, about 43 percent of nursing homes were
cited for at least one IPC deficiency. We also previously reported that, in
each year from 2013 through 2017, the percent of all nursing homes
inspected by state surveyors with an IPC deficiency ranged from 39 to 41
percent.
38
According to most of the state survey agency officials we interviewed and
our review of IPC deficiency narratives written by state surveyors, nursing
homes received IPC deficiencies during the pandemic for failing to follow
basic IPC practices, such as proper handwashing and personal protective
equipment usage, but some state survey officials noted that nursing
homes also received IPC deficiencies for failing to follow COVID-19-
specific practices such as failing to quarantine and isolate COVID-19
positive residents. (See app. II for illustrative examples of IPC
deficiencies.) When examining the severity of the deficiencies cited, we
36
See M. Levere, P. Rowan, A. Wysocki, “The Adverse Effects of the COVID-19
Pandemic on Nursing Home Resident Well-Being,” JAMDA, vol. 22, no. 5 (2021): 948-
954.
37
For this analysis, we analyzed the deficiency code F-880 for nursing homes that were
cited for not meeting federal standards for establishing and maintaining an IPC program.
38
See GAO-20-576R.
Infection Prevention
and Control
Deficiencies
Persisted in Nursing
Homes during the
COVID-19 Pandemic
The Percentage of
Nursing Homes Cited for
Infection Prevention and
Control Deficiencies
during the Pandemic Was
Generally Consistent with
Prior Years

Page 16 GAO-22-105133 Nursing Home Infection Control
found that in 2018 and 2019, only 1 percent of IPC deficiencies were
classified at a high severity where the surveyor determined that residents
were harmed or in immediate jeopardy of being harmed.
39
However,
during the pandemic in 2020 and 2021, this increased to about 8 and 4
percent, respectively.
CMS put greater emphasis on IPC when it temporarily suspended
standard surveys and introduced focused infection control surveys
beginning in March 2020. (See app. III for more information on the
focused infection control survey and the next finding for how it fits in with
other actions CMS took during the pandemic.) While the enhanced
scrutiny of IPC through CMS’s focused infection control survey does not
appear to have resulted in a greater percentage of nursing homes being
cited by surveyors for IPC deficiencies during the pandemic compared to
prior years, the focused infection control surveys were the key source of
IPC deficiencies in 2020.
40
Specifically, our analysis of the CMS data
showed that, prior to the pandemic, the vast majority of IPC deficiencies
were identified during standard surveys (about 84 percent in 2018 and
39
This is consistent with our prior reporting, where we found that, in each year from 2013
through 2017, nearly all IPC deficiencies (about 99 percent in each year) were classified
by surveyors as not severe, meaning the surveyor determined that residents were not
harmed. See GAO-20-576R.
IPC deficiencies were also categorized by scope—whether the incident was an isolated
occurrence, a part of a pattern of behavior, or a widespread behavior. In 2018 and 2019,
about 50 percent of IPC deficiencies cited were categorized as isolated, about 30 percent
categorized as a pattern, and about 14 percent categorized as widespread. In 2020 and
2021, about 35 percent of IPC deficiencies cited were categorized as isolated, about 40
percent were categorized as pattern, and about 20 percent were categorized as
widespread. Percentages do not add to 100 due to rounding.
40
In January 2021 and again in November 2021, CMS gave state survey agencies more
capacity to conduct additional standard surveys by changing the criteria for how often a
focused infection control survey must be conducted, after a year of state survey agencies
mainly conducting the more frequent focused infection control surveys. See Centers for
Medicare & Medicaid Services, COVID-19 Survey Activities, CARES Act Funding,
Enhanced Enforcement for Infection Control Deficiencies, and Quality Improvement
Activities in Nursing Homes, QSO-20-31-ALL (Baltimore, Md.: June 1, 2020) (revised
January 4, 2021) and Centers for Medicare & Medicaid Services, Changes to COVID-19
Survey Activities and Increased Oversight in Nursing Homes, QSO-22-02-ALL (Baltimore,
Md.: Nov. 12, 2021). Nursing homes could be inspected multiple times in a calendar year
with a focused infection control survey, depending on the number of outbreaks. On
average, nursing homes had four focused infection control surveys in 2020 and three in
2021. In each year from 2018 through 2021, nursing homes had, on average, two
complaint or facility-reported incident investigations and one standard survey.

Page 17 GAO-22-105133 Nursing Home Infection Control
2019).
41
In contrast, in 2020, which encompasses the period when
standard surveys were temporarily suspended, the majority of IPC
deficiencies were identified during focused infection control surveys—60
percent in 2020, which decreased to 31 percent in 2021. Further, as the
percentage of IPC deficiencies identified during standard surveys
dropped during the pandemic, the percentage of IPC deficiencies
identified during complaint or facility-reported incident inspections
increased from about 16 percent in 2018 and 2019, to 26 percent in 2020
and 29 percent in 2021. (See fig. 3.)
Figure 3: Type of Survey or Investigation Used by State Survey Agencies to Identify
Infection Prevention and Control Deficiencies, 2018 through 2021
Notes: For 352 of the 34,522 IPC deficiencies cited from 2018 through 2021 (about 1 percent), we
were unable to determine from CMS’s data whether the deficiency was identified during a standard
survey, complaint or facility-reported incident investigation, or focused infection control survey. We
excluded these deficiencies from our percentages.
41
For 352 of the 34,522 IPC deficiencies cited from 2018 through 2021 (about 1 percent),
we were unable to determine from CMS’s data whether the deficiency was identified
during a standard survey, complaint or facility-reported incident investigation, or focused
infection control survey. We excluded these deficiencies from our percentages.

Page 18 GAO-22-105133 Nursing Home Infection Control
CMS’s suspension of standard surveys and shift to prioritizing the new
focused infection control survey in 2020 was a factor contributing to
standard survey backlogs in some states due to the growing number of
nursing homes exceeding the federal standard of 15 months without a
standard survey.
42
According to CMS data, as of April 2022, about 40
percent of nursing homes went at least 16 months without receiving a
standard survey.
43
Our review of CMS data found that about 95 percent of
nursing homes had a standard survey conducted in each of the 2 years
we examined prior to the pandemic. During the pandemic, only 28
percent of nursing homes had a standard survey in 2020 while nearly all
homes had at least one focused infection control survey, resulting in half
as many total deficiencies as prior to the pandemic. In 2021, about 57
percent of nursing homes had a standard survey, and about 80 percent of
nursing homes had at least one focused infection control survey, but the
resulting number of total deficiencies cited by surveyors was still about
one-quarter less than pre-pandemic levels.
44
This may be because the
standard survey provides a comprehensive assessment across multiple
areas of a nursing home’s safety and quality of care, while the focused
infection control survey is more narrowly scoped to assess a nursing
home’s IPC practices in light of COVID-19.
Our analysis of CMS data shows that a smaller percentage of nursing
homes were cited for eight other IPC deficiency codes during the time
42
According to CMS, some state survey agencies had staffing issues during the pandemic
that hindered their ability to conduct standard surveys, including staff reassignments and
retirements, which also contributed to the backlog. For example, CMS officials said that
many states had to pull their surveyors, most of whom were nurses, from their survey
roles and deploy them to provide direct care to community residents or to fill other clinical
roles in response to the pandemic. Also, many state survey agencies saw an increase in
complaint allegations that needed to be investigated, which took resources away from
conducting standard surveys.
43
This is a decrease from May 2021, when the HHS Office of Inspector General reported
that 71 percent of nursing homes had gone at least 16 months without receiving a
standard survey. See HHS Office of Inspector General, States’ Backlogs of Standard
Surveys of Nursing Homes Grew Substantially During the COVID-19 Pandemic, OEI-01-
20-00431 (Washington, D.C.: July 27, 2021). One factor contributing to this decrease
could be the steps CMS announced in November 2021 to assist state survey agencies in
addressing the backlog of standard surveys, such as by revising the criteria for conducting
a focused infection control survey and guidance for resuming standard surveys. See
Centers for Medicare & Medicaid Services, QSO-22-02-ALL (Nov. 12, 2021).
44
The percentage of nursing homes with a complaint or facility-reported incident
investigation was about 53 percent in 2018, about 56 percent in 2019, about 45 percent in
2020, and about 52 percent in 2021.

Page 19 GAO-22-105133 Nursing Home Infection Control
period examined.
45
Specifically, four of these eight IPC deficiency codes
were established by CMS during the pandemic.
46
For example, a
deficiency code for not meeting federal standards for informing residents,
representatives, and families of COVID-19 cases in a nursing home went
into effect in May 2020 and, in 2020 and 2021, less than 3 percent of
nursing homes inspected by surveyors were cited for this deficiency code.
The remaining four IPC deficiency codes were established by CMS in the
years prior to the pandemic. For example, the antibiotic stewardship
program deficiency code went into effect in November 2017 and, from
2018 through 2021, 5 percent or less of the nursing homes inspected by
surveyors were cited for this deficiency code. (See app. IV for additional
data on deficiencies cited.)
Officials from seven of the eight state survey agencies we spoke to said
that persistent IPC challenges faced by nursing homes during the
pandemic, were rooted in staffing challenges, including staffing shortages
and high rates of staff turnover.
47
According to CMS officials, the reasons
for staffing shortages can be complex and unclear, ranging from an
inadequate recruitment pool to management decisions. Officials we
interviewed from four state survey agencies said that, if a nursing home
does not have enough staff, it could be challenging for staff to adhere to
proper IPC practices, such as taking the time to properly put on and
remove personal protective equipment or wash their hands between
caring for multiple residents. Officials from one state survey agency we
interviewed said that staffing shortages have occurred in nursing homes
throughout the pandemic because, for example, employees are out sick.
In addition, officials from four nursing homes we interviewed said that
they have sought to adhere to CDC guidance recommending a dedicated
space in the home, if possible, for residents with confirmed COVID-19
45
These eight other deficiency codes are F-881 for the antibiotic stewardship program, F-
882 for the infection preventionist role, F-883 for influenza and pneumococcal
immunization, F-945 for infection control training, F-884 for reporting to the National
Healthcare Safety Network, F-885 for reporting to residents, representatives, and family,
F-886 for COVID-19 testing for residents and staff, and F-887 for COVID-19
immunizations.
46
See 42 C.F.R. § 483.80(d)(3), (g), (h) (2021).
47
Even before the COVID-19 pandemic, nursing homes have historically struggled with
staffing shortages and high rates of staff turnover. For more, see National Academies of
Sciences, Engineering, and Medicine, The National Imperative to Improve Nursing Home
Quality: Honoring Our Commitment to Nursing Home Residents, Families, and Staff
(Washington, D.C.: The National Academies Press, 2022).
Selected State Officials
Attributed Persistent
Infection Prevention and
Control Challenges to
Staffing Shortages and
High Turnover

Page 20 GAO-22-105133 Nursing Home Infection Control
infections, which has resulted in additional staffing needs.
48
Officials from
seven of the nine nursing homes we spoke with said they have
experienced a staffing shortage during the pandemic.
Officials from five of the state survey agencies we spoke with noted that
there had been a lot of staff turnover during the pandemic, which made it
difficult for a home to ensure that new or temporary staff are trained on
IPC. (In response to the pandemic, CMS gave nursing homes more
flexibility in hiring temporary employees to work as nurse aides by
suspending certain training and certification requirements.
49
) According to
officials from one state survey agency, some of these temporary
employees had never worked in a nursing home before. Officials from
some nursing homes we interviewed also reported using temporary staff
from nurse staffing agencies. Officials we interviewed from three state
survey agencies said that while nursing homes typically do in-service
training for their own permanent staff, they may not have had the time or
resources to provide the same training to temporary staff during the
pandemic, including staff from nurse staffing agencies. Officials from
seven nursing homes we interviewed noted that this was compounded by
the challenges of keeping staff trained on guidance, which officials said
was constantly changing due to the changing circumstances of the
pandemic.
48
CDC guidance specifies that staff should be assigned to work only in this unit when it is
in use and that at a minimum, staff in the COVID-19 unit should include the primary
nursing assistants and nurses assigned to care for these residents. Accessed on
November 8, 2021, https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html.
49
Specifically, from March 2020 through June 2022, CMS waived the requirement that a
nursing home not employ anyone for more than 4 months unless they meet certain
training and certification requirements to address potential staffing shortages in nursing
homes due to the COVID-19 pandemic. See Centers for Medicare & Medicaid Services,
COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers, (Baltimore,
Md.: March 13, 2020) and Centers for Medicare & Medicaid Services, QSO-22-15-NH
(April 7, 2022).
Page 21 GAO-22-105133 Nursing Home Infection Control
Nursing home officials we interviewed from our selected states said that
nursing homes gained valuable knowledge about IPC practices during the
COVID-19 pandemic. For example, nursing home officials said their
understanding of the significance and additional application of basic IPC
practices—such as the importance of proper handwashing and the proper
use of personal protective equipment—was enhanced. Officials from one
nursing home said that, prior to the pandemic, the home would conduct
an annual IPC “boot camp” training but the pandemic taught them that
those IPC skills were easy to forget when they were not constantly put
into practice. An official from another nursing home said that the IPC
lessons that staff learned during the COVID-19 pandemic were applicable
to preventing the spread of other types of infections.
Nursing home officials we interviewed also described learning new
COVID-19 specific practices, such as how to conduct on-site testing, set
up quarantine and isolation units, and screen visitors and staff. Officials
from one nursing home described developing a process for swabbing and
testing nearly 150 staff members for COVID-19 twice a week. Officials
from another nursing home said they learned how to work with the design
of their building to locate adequate quarantine and isolation spaces.
Officials from two other nursing homes described IPC practices they
implemented during the pandemic that they hoped to continue going
forward. For example, officials from one nursing home said that when the
pandemic ends they plan to continue the visitor and staff symptom
screening they put in place for COVID-19 to prevent the spread of
infections.
Our review of agency documentation and interviews with agency officials
show that CMS and CDC took numerous actions to improve infection
prevention and control both prior to and during the pandemic. For
example, prior to the pandemic, CMS required nursing homes to
designate an infection preventionist on staff and, during the pandemic,
CMS and CDC provided infection prevention resources to nursing homes.
(See app. V for a full list of IPC actions identified by CMS and CDC.)
Despite Challenges,
Nursing Home Officials
from Selected States
Reported Gaining
Knowledge about Infection
Prevention and Control
Practices during the
Pandemic
CMS and CDC Took
Actions to Strengthen
Infection Prevention
and Control but
Should Do More
CMS and CDC Took
Numerous Actions on
Infection Prevention and
Control

Page 22 GAO-22-105133 Nursing Home Infection Control
Examples of actions CMS and CDC took prior to the COVID-19 pandemic
include the following:
• Required designated infection preventionist. CMS updated IPC
requirements to include the requirement that nursing homes designate
at least one infection preventionist to oversee the facility’s IPC
program, effective beginning November 2019.
• Developed infection preventionist training. To support the infection
preventionist requirement, CMS, in consultation with CDC, developed
a free online infection preventionist training program that was
available to nursing homes as of March 2019.
50
The specialized
training provided content covering a range of IPC topics to prepare
infection preventionists for their role.
• Conducted IPC pilot program and released Infection Control
Worksheet tool. To help assess and prevent infections in nursing
homes, CMS, in consultation with CDC, conducted a 3-year IPC pilot
project from fiscal year 2016 through 2018, which used a worksheet
tool, developed with CDC and expert input, to identify gaps in nursing
home IPC practices and guide assistance to address those gaps.
51
CMS released the worksheet as an IPC self-assessment tool to
nursing homes in November 2019.
52
Examples of key actions CMS and CDC took during the pandemic include
the following:
• Initiated focused infection control surveys. In March 2020, CMS
made key changes in how it oversees nursing homes by requiring
state survey agencies to conduct a new survey type known as the
focused infection control survey that assessed IPC-related
requirements specific to COVID-19, such as adherence to visitor
50
See Centers for Medicare & Medicaid Services, Specialized Infection Prevention and
Control Training for Nursing Home Staff in the Long-Term Care Setting is Now Available,
QSO-19-10-NH (Baltimore, Md.: March 11, 2019).
51
See Centers for Medicare & Medicaid Services, Infection Control Pilot Project, S&C-16-
05-ALL (Baltimore, Md.: Dec. 23, 2015).
52
For the pilot, the new survey tool was used for educational purposes rather than to
assess compliance with existing IPC requirements. After the surveyors assessed the
participating nursing homes’ IPC practices, the nursing homes were provided with
technical assistance based on the survey’s results. See Centers for Medicare & Medicaid
Services, S&C-16-05-ALL (Dec. 23, 2015).
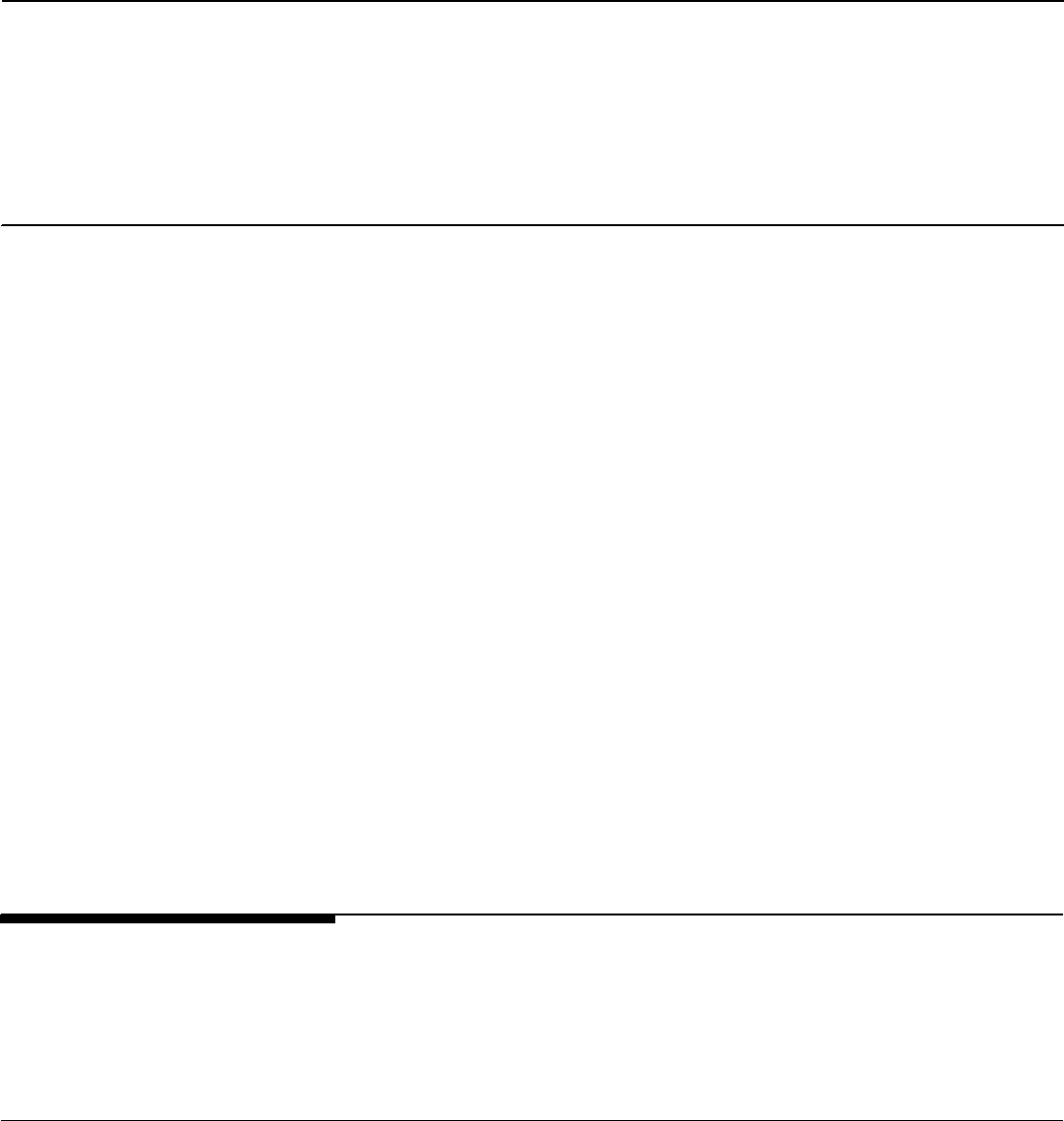
The infection preventionist role
The infection preventionist is a nursing home
employee with training in infection prevention
and control who is responsible for the home’s
program for preventing, identifying, reporting,
investigating, and controlling infections and
communicable diseases. Beginning
November 2019, the Centers for Medicare &
Medicaid Services (CMS) required all nursing
homes to designate one or more infection
preventionists who has completed specialized
training in infection prevention and control and
who works at the nursing home at least part-
time. Some of the responsibilities of the
infection preventionist may include contact
tracing during an infectious disease outbreak,
reporting surveillance data, and educating
staff on proper adherence to infection
prevention and control practices.
Source: GAO summary of CMS and the Centers for Disease
Control and Prevention documents. | GAO-22-105133

Page 23 GAO-22-105133 Nursing Home Infection Control
screening and personal protective equipment protocols.
53
(See app.
III.)
• Restricted visitation and group activities. In March 2020, to limit
the transmission of COVID-19, CMS temporarily restricted visitation
from all visitors and non-essential health care personnel, except for
certain compassionate care situations and suspended group
activities.
54
In November 2021, CMS lifted these restrictions.
55
• Developed IPC-specific training and technical assistance. CMS
and CDC developed training and technical assistance resources to
help nursing homes implement IPC practices. For example, in May
2020, CMS released a toolkit of COVID-19 best practices.
56
In June
2020, CMS deployed a network of quality improvement organizations
to provide technical assistance to approximately 3,000 low performing
nursing homes with a history of infection control challenges.
57
Beginning in July 2020, CDC deployed “strike teams” of infection
prevention and public health professionals to nursing homes facing
53
CMS continued to require state survey agencies to conduct high-priority complaint
investigations, such as those conducted in response to alleged abuse or neglect. See
Centers for Medicare & Medicaid Services, Prioritization of Survey Activities, QSO-20-20-
ALL (Baltimore, Md.: March 20, 2020).
54
These restrictions included ombudsmen, which are advocates for nursing home
residents. These restrictions were later clarified to allow certain conditions for visitation,
such as to allow residents access to long-term care ombudsmen. See Centers for
Medicare & Medicaid Services, QSO-20-14-NH (Mar. 13, 2020 revision) and Centers for
Medicare & Medicaid Services, Nursing Home Five Star Quality Rating System Updates,
Nursing Home Staff Counts, Frequently Asked Questions, and Access to Ombudsman,
QSO-20-28-NH (Baltimore, Md.: April 24, 2020 and Jul. 9, 2020 revision). After the initial
restrictions, CMS made changes to its visitation guidance multiple times during the
pandemic to allow increased visitation and group activities. See Centers for Medicare &
Medicaid Services QSO-20-39-NH (Sept. 17, 2020), revised March 10, 2021 and April 27,
2021.
55
See Centers for Medicare & Medicaid Services, QSO-20-39-NH (Nov. 12, 2021
revision).
56
See Centers for Medicare & Medicaid Services, CMS Issues Nursing Homes Best
Practices Toolkit to Combat COVID-19, May 13, 2020, accessed April 18, 2022,
https://www.cms.gov/newsroom/press-releases/cms-issues-nursing-homes-best-
practices-toolkit-combat-covid-19.
57
See Centers for Medicare & Medicaid Services, QSO-20-31-ALL (June 1, 2020).

Page 24 GAO-22-105133 Nursing Home Infection Control
challenges with infection control.
58
In August 2020, CMS released
online IPC training courses developed in consultation with CDC.
• Mandated COVID-19 surveillance reporting. In May 2020, CMS
required nursing homes to report data at least weekly through CDC’s
National Healthcare Safety Network on COVID-19 cases and deaths
among residents and staff, personal protective equipment supplies,
access to testing, and staff shortages, among other things.
59
• Increased IPC enforcement actions. In June 2020, CMS increased
financial and other penalties, such as requiring directed plans of
correction, for nursing home noncompliance with IPC requirements
and made enforcement actions more significant for nursing homes
with a history of past infection control deficiencies.
60
58
The strike teams identified challenges related to staffing, personal protective equipment
supplies, COVID-19 testing, and infection prevention and control measure implementation.
See L. Anderson et al., “Protecting Nursing Home Residents from COVID-19: Federal
Strike Team Findings and Lessons Learned,” New England Journal of Medicine Catalyst
(June 28, 2021).
59
85 Fed. Reg. 27,550, 27,627 (May 8, 2020) (codified at 42 C.F.R. § 483.80(g)). Until
December 31, 2024, the new requirement provides for these data to be reported at the
federal level through CDC’s National Healthcare Safety Network and to be updated and
publicly reported. Prior to this reporting requirement, state and local health departments
may have required nursing homes to report certain COVID-19 related information to them
as part of their infectious disease surveillance programs. See 42 C.F.R. § 483.80(a)(2)(ii)
(2021). In May 2021, CMS also required nursing homes to report COVID-19 vaccine and
therapeutics treatment information to the CDC’s National Healthcare Safety Network.
Medicare and Medicaid Programs; COVID–19 Vaccine Requirements for Long-Term Care
Facilities and Intermediate Care Facilities for Individuals with Intellectual Disabilities
Residents, Clients, and Staff, 86 Fed. Reg. 26,306, 26,336 (May 13, 2021) (codified at 42
C.F.R. § 483.80(g)(1)(viii)-(ix)).
60
As part of these efforts, CMS encouraged state survey agencies to develop and issue to
noncompliant nursing homes directed plans of correction, as their enforcement action, in
which state survey agencies specify actions a nursing home must take to address
infection control deficiencies, such as obtaining further IPC training or hiring an IPC
consultant. See Centers for Medicare & Medicaid Services, QSO-20-31-ALL (June 1,
2020).
On February 28, 2022, the White House announced that it would lead further efforts to
improve quality and safety in nursing homes through enforcement actions. For example, it
announced a commitment to hold poorly performing nursing homes accountable for
improper and unsafe care by expanding financial penalties and other sanctions and
including more nursing homes in an enhanced oversight program targeting the poorest
performers.

Page 25 GAO-22-105133 Nursing Home Infection Control
Nursing home and state survey agency officials reported to us what they
believed were advantages and disadvantages for selected IPC actions
taken before and during the pandemic. For example, state survey agency
and nursing home officials told us that CMS’s requirement to designate
an infection preventionist was crucial to nursing homes during the
COVID-19 pandemic. See table 1.
Stakeholders Reported
Advantages and
Disadvantages of CMS
and CDC Infection
Prevention and Control
Actions

Page 26 GAO-22-105133 Nursing Home Infection Control
Table 1: Selected Federal Infection Prevention and Control (IPC) Actions and Examples of Stakeholder-Reported Perspectives
on Advantages and Disadvantages
Action
Description
Advantages
Disadvantages
Required designated
infection preventionist
The Centers for Medicare &
Medicaid Services (CMS)
updated IPC requirements to
include the designation of
infection preventionists.
•
Critical role in nursing homes
during the pandemic (eight of
nine nursing homes and five of
eight state survey agencies)
•
Requirement needs
strengthening to ensure
sufficient infection preventionist
staffing levels (two of nine
nursing homes and three of
eight state survey agencies)
• Difficult to hire or retain infection
prevention professionals in order
to comply (one of nine nursing
homes and two of eight state
survey agencies)
Developed infection
preventionist training
CMS, in consultation with the
Centers for Disease Control
and Prevention (CDC),
developed infection
preventionist training in
preparation for the infection
preventionist requirement.
•
Training is helpful,
comprehensive (six of nine
nursing homes and three of
eight state survey agencies)
•
Training is time intensive (one of
nine nursing homes and two of
eight state survey agencies)
• Training curriculum is limited,
other training opportunities
needed (one of nine nursing
homes and four of eight state
survey agencies)
Conducted IPC pilot
program and released
Infection Control
Worksheet
CMS, in consultation with
CDC, conducted a pilot from
fiscal year 2016 through 2018
to help prevent the spread of
infections in nursing homes.
CMS released the Infection
Control Worksheet for nursing
homes.
Generally, the information we gathered from stakeholders indicated limited
awareness of the worksheet as a tool to help nursing homes.
Initiated focused
infection control surveys
CMS developed the focused
infection control survey to
assess IPC-related
requirements specific to
COVID-19.
•
Helps improve IPC practices
(seven of nine nursing homes
and four of eight state survey
agencies)
•
Punitive rather than helpful
approach (five of nine nursing
homes and one of eight state
survey agencies)
• Frequent and distracting from
resident care (four of nine
nursing homes and five of eight
state survey agencies)
• Guidance unclear (three of nine
nursing homes and eight of eight
state survey agencies)
• Contributed to state survey
agencies’ backlogs of standard
surveys (six of eight state survey
agencies)
Restricted visitation and
suspended group
activities
CMS temporarily restricted
nursing home visitation and
suspended group activities.
•
Necessary to keep residents and
staff safe (three of nine nursing
homes and four of eight state
survey agencies)
•
Isolated residents and resulted
in some mental or physical
declines in health (six of nine
nursing homes and five of eight
state survey agencies)

Page 27 GAO-22-105133 Nursing Home Infection Control
Action
Description
Advantages
Disadvantages
Developed IPC-specific
training and technical
assistance
CMS and CDC developed
training and technical
assistance resources to help
nursing homes implement
IPC practices.
•
Helps improve IPC practices (six
of nine nursing homes and six of
eight state survey agencies)
•
Content basic or not timely and
accessible (three of nine nursing
homes and six of eight state
survey agencies)
Mandated COVID-19
surveillance reporting
CMS required nursing homes
to report to CDC weekly
surveillance data, such as
COVID-19 cases and deaths.
•
Useful for directing resources
and policy improvements (one of
nine nursing homes and four of
eight state survey agencies)
•
Weekly reporting burden (five of
nine nursing homes and four of
eight state survey agencies)
• Lack of clear training and
instructions (three of nine
nursing homes)
Increased IPC
enforcement actions
CMS increased financial and
other penalties for nursing
home noncompliance with
IPC requirements.
•
Incentivizes improvements (five
of eight state survey agencies)
• Provides more effective options,
such as directed plans of
correction, for system change
(four of eight state survey
agencies)
•
Overly punitive during a
pandemic (four of nine nursing
homes and five of eight state
survey agencies)
Source: GAO interviews with selected state survey agency and nursing home officials in eight states. | GAO-22-105133
In our review of CMS IPC oversight, we identified areas where CMS could
take more actions to strengthen oversight of IPC in nursing homes.
Specifically, we found that CMS could take steps to strengthen both the
role of the infection preventionist in nursing homes and IPC guidance.
As previously described, most nursing home and state survey agency
officials we interviewed indicated that CMS’s requirement that nursing
homes have an infection preventionist was critical to helping nursing
homes address IPC challenges during the pandemic. Some of these
officials, representing two very distinct perspectives, suggested CMS take
actions to clarify and strengthen requirements for the role. We identified
two ways that CMS could strengthen its oversight of the infection
preventionist role: (1) establish minimum training standards and (2)
collect and use infection preventionist staffing data to assess the
sufficiency of the current staffing requirement.
Establish minimum infection preventionist training standards. We
found that training for nursing home infection preventionists is
inconsistent because CMS has not specified the minimum training that
infection preventionists need to receive so that they can be effective
performing their role in nursing homes.
As part of its 2016 regulatory update of nursing home requirements, CMS
began requiring nursing homes to designate an infection preventionist by
CMS Has Opportunities to
Strengthen Infection
Prevention and Control
Oversight
Strengthen Oversight of the
Infection Preventionist Role

Page 28 GAO-22-105133 Nursing Home Infection Control
November 28, 2019, and required infection preventionists to have
completed “specialized training in IPC.” However, the requirement lacks
specificity about what, at a minimum, the specialized training should
comprise. According to CMS, the agency does not set minimum training
requirements for other types of nursing home personnel and expects
nursing homes to provide the amount of training needed to ensure staff
have the skills to do their jobs.
61
One state survey agency official told us
that nursing homes are using a variety of training programs that are not
equally rigorous to meet the CMS requirement, each with different
curricula and covering different topics. Therefore, the official saw a need
for standardizing infection preventionist training programs.
62
Further,
survey results from a 2018 study found that only 39 percent of nursing
homes surveyed reported that their infection preventionists had
completed specialized training in IPC.
63
Additionally, CMS, CDC, and
state survey agency officials from some of our selected states identified
noticeable gaps in the skills of nursing home infection preventionists
during the pandemic, with CMS and CDC officials noting that some
infection preventionists were unable to develop strategies for addressing
common IPC practice errors, such as with hand hygiene. According to
CMS and CDC officials, there are numerous trainings available for
infection preventionists, including a comprehensive training program
developed by CMS, in consultation with CDC, in March of 2019.
61
CMS said that the agency requires nurse aides to complete 75 hours of training,
because this minimum initial training standard is established in statute. See 42 U.S.C. §§
1395i-3(f)(2)(A)(i)(II), 1396r(f)(2)(A)(i)(II).
62
Further, CDC officials said that historically there have been limited training courses
available for a nursing home infection preventionist to obtain nursing-home specific IPC
knowledge because many of the available training programs were not initially designed for
nursing home settings. CDC officials also noted that since 2016, when the infection
preventionist requirement was published, multiple courses have been developed and that
these courses may be variable in terms of training time and topics since they were not
developed in response to required specifications.
In addition, studies have found a lack of training among the personnel responsible for
infection prevention and control in nursing homes. See National Academies of Sciences,
Engineering, and Medicine, The National Imperative to Improve Nursing Home Quality:
Honoring Our Commitment to Nursing Home Residents, Families, and Staff (Washington,
D.C.: The National Academies Press, 2022).
63
P. Stone et al., “Nursing Home Infection Control Program Characteristics, CMS
Citations, and Implementation of Antibiotic Stewardship Policies: A National Study,”
INQUIRY: The Journal of Health Care Organization, Provision, and Financing, vol. 55
(2018) 1-7.

Page 29 GAO-22-105133 Nursing Home Infection Control
Establishing minimum training requirements would be consistent with
federal standards for internal control that call for management to set clear
expectations of competence for key roles, such as the role of the infection
preventionist.
64
CMS planned to issue additional guidance to clarify the
role of the infection preventionist, which could include more information
about the minimum training infection preventionists need, but CMS
officials told us that the agency has delayed issuance multiple times due
to the COVID-19 pandemic. In June 2022, CMS released an advance
copy of guidance, which clarifies the role of the infection preventionist but
does not clarify infection preventionists’ minimum training requirements,
such as how many hours of training infection preventionists must
complete.
65
Until CMS establishes minimum training standards for
infection preventionists, nursing homes may not know which training
programs are adequate and required for preparing their infection
preventionists, and the skills of infection preventionists may not be
adequate to allow them to effectively perform their role.
Collect and use infection preventionist staffing data. We found that
CMS does not collect staffing data on infection preventionists in its
staffing data system as it does for other positions. As a result, the agency
lacks information it could use to assess whether CMS’s minimum staffing
standard for a part-time infection preventionist is sufficient to address
infection risks to both residents and staff in all nursing homes.
Some nursing home and state survey agency officials from our selected
states told us that many part-time infection preventionists do not have
sufficient time to conduct the IPC tasks that could limit the risk of
infections. Specifically, we heard from some of the nursing homes and
state survey agency officials that having only a part-time infection
preventionist was not sufficient for some homes. Infection preventionists
we interviewed from five of nine nursing homes in our review were staff
members who shared other significant and demanding roles, such as
serving as the Director of Nursing, and, as a result, some were hampered
in their ability to carry out all of their infection prevention responsibilities.
For example, one nursing home infection preventionist said that, because
she also serves as the facility’s assistant director of nursing, often her
infection preventionist role is a “second thought assignment.”
64
GAO-14-704G.
65
See Centers for Medicare & Medicaid Services, Revised Long-Term Care Surveyor
Guidance, QSO-22-19-NH (Baltimore, Md.: June 29, 2022).

Page 30 GAO-22-105133 Nursing Home Infection Control
When discussing the requirement that nursing homes must have at least
one part-time infection preventionist on staff, CMS officials told us the
requirement was designed to allow nursing homes flexibility to determine
the amount of time needed for an infection preventionist to effectively
oversee the facility’s IPC program. In June 2022, CMS released an
advance copy of guidance, which notes that, while the CMS requirement
is to have an infection preventionist at least part-time, nursing homes are
responsible for an effective IPC program and should ensure the role of
the infection preventionist is tailored to meet the nursing home’s needs.
66
However, nursing home and state survey agency officials from four states
in our review told us that nursing homes do not always dedicate funding
to hire infection preventionists beyond the minimum required, regardless
of the need. Finally, the CMS Coronavirus Commission for Safety and
Quality in Nursing Homes’ report from September 2020, highlighted
findings that part-time infection preventionists often cannot adequately
respond to the demands of the COVID-19 pandemic and recommended
that CMS determine whether or under what circumstances nursing homes
should have more than one part-time preventionist.
67
To the extent that CMS’s current infection preventionist requirement may
be inadequate for some nursing homes, it poses a potential risk to CMS’s
goal of ensuring quality care for nursing home residents. Addressing risk
is consistent with federal standards for internal control that call for
management to identify, analyze, and respond to risks by estimating their
effect on achieving a defined objective.
68
CMS could begin to assess this
risk with data on preventionist staffing levels across the nursing homes it
oversees. It could require nursing homes to submit staffing data on
infection preventionists through its Payroll Based Journal System, as it
does with other staffing positions, which would result in the agency having
comprehensive data on the number of hours infection preventionists are
66
See Centers for Medicare & Medicaid Services, QSO-22-19-NH (June 29, 2022).
67
Specifically, the commission recommended that CMS establish an evidence-based
standard for an infection preventionist educator full-time equivalent to bed ratio, among a
number of other recommended steps CMS could take to strengthen the role of infection
preventionists in nursing homes. See MITRE, Coronavirus Commission on Safety and
Quality in Nursing Homes, Commission Final Report (McLean, Va.: The MITRE
Corporation, 2020). This report was written for CMS under a government contract.
68
GAO-14-704G.

Page 31 GAO-22-105133 Nursing Home Infection Control
paid to work each day.
69
CMS could then use these staffing data to
examine what level of infection preventionist staffing is needed based on
nursing home size and the complexity of resident care needs. The agency
could also use the data to compare the relationship, if any, between IPC
deficiencies and infection preventionist staffing levels. CMS does not
currently collect the infection preventionist staffing data in this way
because the infection preventionist role was created after the Payroll
Based Journal System was rolled out in 2015.
Collecting and utilizing quality information to inform agency decisions is
consistent with federal standards for internal control to use quality
information to achieve objectives.
70
Having comprehensive data on
infection preventionist staffing levels across nursing homes would allow
the agency to begin assessing whether the standard is sufficient for
protecting nursing home residents and staff or whether it needs to be
modified.
As previously described, some nursing home and state survey agency
officials from our selected states indicated that the guidance issued by
CMS for some IPC oversight actions was unclear and, in some situations,
resulted in concerns about the enforcement actions taken against nursing
homes. We identified how CMS could strengthen its guidance around IPC
oversight actions by providing additional guidance to help nursing homes
and state survey agencies to assess IPC practices.
We found that CMS’s State Operations Manual—the key guidance state
survey agencies use for conducting nursing home surveys—does not
contain important IPC-related guidance. Specifically, as of May 2022, the
State Operations Manual does not have examples that surveyors can use
to assess the scope and severity of deficiencies applicable to COVID-19-
related IPC requirements. For example, the scope and severity examples
for the IPC deficiency code (F-880) did not include examples related to
the use of personal protective equipment, cohorting (or grouping)
69
The Payroll Based Journal System was developed in 2015 in response to the Patient
Protection and Affordable Care Act, which required CMS to establish a national system to
collect and report payroll data on nurse staffing hours. Pub. L. No. 111-148, §§ 6103,
6106, 124 Stat. 119, 704 (2010) (codified at 42 U.S.C. §§ 1320-7j(g), 1395i-3(i)(1)(A)(i)),
1396r(i)(1)(A)(i)). The system allows the agency to collect staffing data on a regular and
more frequent basis than previously, when the data were reported by the homes during
surveys, and the system allows the data to be auditable to ensure accuracy.
70
GAO-14-704G.
Strengthen Infection
Prevent
ion and Control
Guidance

Page 32 GAO-22-105133 Nursing Home Infection Control
residents and staff to limit opportunities for transmission, and
quarantining, that may be more applicable to stopping the spread of
outbreaks from COVID-19 and other respiratory diseases spread by
droplets and aerosols (e.g., influenza).
71
According to CMS, routine
updates to the State Operations Manual have not been made during the
pandemic due to the temporary nature of certain guidance and the need
for issuing more frequent, immediate updates, which CMS released
through memoranda. In June 2022, while this report was with the agency
for review and comment, CMS released an advance copy of the State
Operations Manual to provide additional guidance to state surveyors for
IPC-related deficiencies, including additional scope and severity
classification examples, but these examples were not specific to COVID-
19 or other types of respiratory diseases. Without COVID-19-relevant
examples for scope and severity classification, some state survey
agencies told us they are sometimes uncertain about how to inspect
nursing homes for adherence to COVID-19 specific requirements, which
officials say can lead to surveyors applying these requirements
inconsistently.
Clarifying its guidance for surveyors would be consistent with CMS’s
State Operations Manual, which states that CMS is responsible for
“conveying operational instructions and official interpretations of policy.”
72
It would also be consistent with federal standards for internal control that
indicate management should communicate the necessary quality
information to achieve its objectives.
73
By providing examples of scope
and severity determinations for IPC related issues in the State Operations
Manual, CMS can help ensure that state survey agencies are better able
71
While Appendix PP of the State Operations Manual provides examples of non-
compliance with precautions around topics such as bloodborne infections, gastrointestinal
illness, and the handling of soiled linens during scabies or head lice outbreaks, the manual
does not contain examples of scope and severity categorization for deficiencies related to
masking, cohorting and quarantining, or other precautions that may be more applicable to
COVID-19 or other respiratory diseases such as influenza transmission. See Centers for
Medicare & Medicaid Services, State Operations Manual, Appendix PP—Guidance to
Surveyors for Long Term Care Facilities (Baltimore, Md.: November 22, 2017). In June
2022, CMS released an advance copy of Appendix PP that will go into effect on October
24, 2022. See Centers for Medicare & Medicaid Services, QSO-22-19-NH (June 29,
2022).
72
Centers for Medicare & Medicaid Services, State Operations Manual, Chapter 1—
Program Background and Responsibilities (Baltimore, Md.: October 3, 2014).
73
GAO-14-704G.

Page 33 GAO-22-105133 Nursing Home Infection Control
to understand and uphold the requirements for managing COVID-19 and
other infectious diseases.
The COVID-19 pandemic has not only led to high rates of severe illness
and death in the nation’s nursing homes, but it also contributed to
worsened mental and physical health among residents and highlighted
persistent problems with infection prevention and control. While CMS and
CDC have taken important actions to try to improve nursing home
infection prevention and control both prior to and during the COVID-19
pandemic, there is more CMS should do. First, CMS should do more to
strengthen oversight of the role of the infection preventionist, a position
whose creation was reported to be critical for helping nursing homes
during the pandemic. Specifically, until CMS sets minimum training
standards for infection preventionists, nursing homes will not know which
training programs are adequate for preparing their infection
preventionists, and the skills of infection preventionists may not be
adequate to allow them to effectively perform their role. Similarly, until
CMS collects and uses infection preventionist staffing data, the agency
will lack information critical to understanding whether infection
preventionists are dedicating enough time to IPC to meet the risks of
infectious disease in nursing homes. Finally, CMS should clarify its IPC
guidance to nursing homes and state survey agencies. Specifically, until
CMS clarifies guidance on the scope and severity examples for IPC
deficiencies specific to COVID-19 and other respiratory diseases, state
survey agencies will continue to face uncertainty about how to inspect
nursing homes for adherence to IPC requirements.
We are making the following three recommendations to the Administrator
of CMS to:
1) Establish minimum infection preventionist training standards.
(Recommendation 1)
2) Collect infection preventionist staffing data and use these data to
determine whether the current infection preventionist staffing requirement
is sufficient. (Recommendation 2)
3) Provide additional guidance in the State Operations Manual on making
scope and severity determinations for IPC-related deficiencies.
(Recommendation 3)
Conclusions
Recommendations for
Executive Action

Page 34 GAO-22-105133 Nursing Home Infection Control
We provided a draft of this report to HHS for review and comment. In its
written comments, printed in appendix VI, HHS agreed with the first of our
three recommendations, but did not state whether the department agreed
or disagreed with our other two recommendations.
Specifically, HHS concurred with our first recommendation and noted that
CMS will consider this recommendation when proposing new
requirements through the rulemaking process.
Regarding our second recommendation, while HHS did not specifically
state whether it agreed or disagreed, the department said that CMS will
consider this recommendation when proposing new requirements through
the rulemaking process. Further, HHS said that CMS will evaluate the
feasibility of collecting infection preventionist staffing data and take
appropriate actions based on this evaluation.
Regarding our third recommendation, HHS did not state whether it agreed
or disagreed, but the department noted that it believes that CMS
addressed this recommendation prior to GAO’s report publication and
therefore requested that GAO remove this recommendation. In June
2022, while a draft of this report was with HHS for review and comment,
CMS released an advance copy of revised guidance, including revisions
to sections of the State Operations Manual relevant to this
recommendation. CMS stated that the agency believes this revised
guidance addresses this recommendation. In response, we updated our
report to reflect this revised guidance. We acknowledge that the revisions,
scheduled to go into effect in October 2022 provide needed additional
guidance on determining the scope and severity of IPC-related
deficiencies. However, none of the revised scope and severity examples
relate to stopping the spread of outbreaks from COVID-19 or other
respiratory diseases spread by droplets and aerosols (e.g., influenza), as
we describe in our report. For example, as we note in our report, none of
the examples in the prior guidance or the revised guidance relate to the
use of personal protective equipment, cohorting residents and staff, or
quarantining residents to limit opportunities for transmission. While we
recognize that CMS has taken some important steps toward addressing
the clarity of the scope and severity examples in the recent update, we
maintain the importance of having examples related to COVID-19 or
respiratory diseases more generally in this guidance. In addition, HHS
also provided technical comments, which we incorporated as appropriate.
Agency Comments
and Our Evaluation

Page 35 GAO-22-105133 Nursing Home Infection Control
We are sending copies of this report to the appropriate congressional
committees, the Secretary of HHS, and other interested parties. In
addition, the report is available at no charge on the GAO website at
http://www.gao.gov.
If you or your staff have any questions about this report, please contact
me at (202) 512-7114 or at [email protected]. Contact points for our
Offices of Congressional Relations and Public Affairs may be found on
the last page of this report. GAO staff who made key contributions to this
report are listed in Appendix VII.
John E. Dicken
Director, Health Care

Page 36 GAO-22-105133 Nursing Home Infection Control
List of Addressees
The Honorable Patrick Leahy
Chairman
The Honorable Richard Shelby
Vice Chairman
Committee on Appropriations
United States Senate
The Honorable Ron Wyden
Chairman
The Honorable Mike Crapo
Ranking Member
Committee on Finance
United States Senate
The Honorable Patty Murray
Chair
The Honorable Richard Burr
Ranking Member
Committee on Health, Education, Labor, and Pensions
United States Senate
The Honorable Gary C. Peters
Chairman
The Honorable Rob Portman
Ranking Member
Committee on Homeland Security and Governmental Affairs
United States Senate
The Honorable Rosa L. DeLauro
Chair
The Honorable Kay Granger
Ranking Member
Committee on Appropriations
House of Representatives

Page 37 GAO-22-105133 Nursing Home Infection Control
The Honorable Frank Pallone, Jr.
Chairman
The Honorable Cathy McMorris Rodgers
Republican Leader
Committee on Energy and Commerce
House of Representatives
The Honorable Bennie G. Thompson
Chairman
The Honorable John Katko
Ranking Member
Committee on Homeland Security
House of Representatives
The Honorable Carolyn B. Maloney
Chairwoman
The Honorable James Comer
Ranking Member
Committee on Oversight and Reform
House of Representatives
The Honorable Richard E. Neal
Chairman
The Honorable Kevin Brady
Republican Leader
Committee on Ways and Means
House of Representatives
The Honorable Michael F. Bennet
United States Senate

Appendix I: Related GAO Products on COVID-
19 in Nursing Homes
Page 38 GAO-22-105133 Nursing Home Infection Control
Health Care Capsule: Improving Nursing Home Quality and Information.
GAO-22-105422. Washington, D.C.: January 14, 2022.
COVID-19: Continued Attention Needed to Enhance Federal
Preparedness, Response, Service Delivery, and Program Integrity.
(Nursing Homes Enclosure). GAO-21-551. Washington, D.C.: July 19,
2021.
COVID-19: Most Homes Had Multiple Outbreaks and Weeks of Sustained
Transmission from May 2020 through January 2021. GAO-21-367.
Washington, D.C.: May 19, 2021.
COVID-19: Sustained Federal Action is Crucial as Pandemic Enters its
Second Year. (Nursing Homes Enclosure). GAO-21-387. Washington,
D.C.: March 31, 2021.
COVID-19 in Nursing Homes: HHS Has Taken Steps in Response to
Pandemic, but Several GAO Recommendations Have Not Been
Implemented. GAO-21-402T. Washington, D.C.: March 17, 2021.
COVID-19: Critical Vaccine Distribution, Supply Chain, Program Integrity,
and Other Challenges Require Focused Federal Attention. (Nursing
Homes Enclosure). GAO-21-265. Washington, D.C.: January 28, 2021.
COVID-19: Urgent Actions Needed to Better Ensure an Effective Federal
Response. (Nursing Homes Enclosure). GAO-21-191. Washington, D.C.:
November 30, 2020.
COVID-19: Federal Efforts Could Be Strengthened by Timely and
Concerted Actions. (Nursing Homes Enclosure). GAO-20-701.
Washington, D.C.: September 21, 2020.
COVID-19: Opportunities to Improve Federal Response and Recovery
Efforts. (Nursing Homes Enclosure). GAO-20-625. Washington, D.C.:
June 25, 2020.
Infection Control Deficiencies Were Widespread and Persistent in Nursing
Homes Prior to COVID-19 Pandemic. GAO-20-576R. Washington, D.C.:
May 20, 2020.
Appendix I: Related GAO Products on
COVID-19 in Nursing Homes

Appendix II: Examples of Infection Prevention
and Control Deficiencies Cited in Nursing
Homes during the Pandemic
Page 39 GAO-22-105133 Nursing Home Infection Control
Infection prevention and control (IPC) practices can be critical to
preventing the spread of infectious diseases, including those specific to
COVID-19. We reviewed examples of IPC deficiency narratives written by
state surveyors to illustrate IPC deficiencies from different time points
during the pandemic.
Table 2: Illustrative Examples of Narratives from Infection Prevention and Control Deficiencies Cited in Nursing Homes during
the Pandemic
Narrative details
Month and year
survey was
conducted
State surveyors observed that high-touch surfaces were not being disinfected and that disinfecting supplies
were not readily available for staff use. In addition, surveyors observed certified nursing assistants and a
nurse in a nursing home not properly wearing personal protective equipment. Specifically, they observed
these staff failing to change or properly wear personal protective equipment between residents with known or
suspected COVID-19, such as two certified nursing assistants who did not change their gowns after providing
care to a resident on droplet precautions. Two staff members indicated to surveyors that they had not been
given any guidance on how long to wear personal protective equipment and when to change it.
July 2020
State surveyors observed staff members in a nursing home having direct contact with residents across both
the COVID-19 negative and positive units. The surveyors also learned that the nursing home had not
previously quarantined any residents after a known exposure to a COVID-19 positive roommate. In addition,
surveyors learned that the infection preventionist continued to work in the facility and have direct contact with
multiple residents in her role as a charge nurse after testing positive for COVID-19. She was immediately sent
home after testing positive, but then she was directed to return to work the next day by the administration and
continued to work her schedule. Further, surveyors observed challenges with personal protective equipment.
They observed a certified nursing assistant provide personal care and assistance to several residents on the
COVID-19 positive unit wearing a jumpsuit, instead of a gown, that she did not change between residents.
She told the surveyors that she had been provided with the jumpsuit by the home and had been wearing it for
several days. She did not remove the jumpsuit prior to leaving the nursing home at the end of her shift and
would instead remove it on her porch and leave it there until her next shift. Then, she would clean it with
disinfecting spray before putting it back on and returning to the home for her shift.
Sept. 2020
State surveyors observed newly admitted residents were not being quarantined from other residents at a
nursing home because, according to the Director of Nursing, there were challenges with space at the home. In
addition, staff failed to properly use full personal protective equipment. The surveyors observed a certified
nursing assistant coming out of a resident’s room with her facemask around her chin and wearing eyeglasses
with no face shield or goggles.
Feb. 2021
State surveyors observed certified nursing assistants in a nursing home assisting residents without performing
any hand hygiene between residents. One certified nursing assistant was observed assisting a resident with
adjusting a wheelchair and a bed side table. Then, she removed the resident’s slice of bread from its wrapping
with her bare hands and spread butter on the bread without performing any hand hygiene. Another certified
nursing assistant did not perform hand hygiene when passing out lunch trays and setting up tray tables
between residents. The certified nursing assistant said that she knew she should wash her hands between
residents but she was trying to pass out the trays faster.
April 2021
Source: GAO analysis of Form-2567 deficiency narrative reports from the Centers for Medicare & Medicaid Services (CMS). | GAO-22-105133
Appendix II: Examples of Infection
Prevention and Control Deficiencies Cited in
Nursing Homes during the Pandemic

Appendix III: Types of Surveys and
Investigations to Assess Whether Nursing
Homes Are Meeting Federal Standards
Page 40 GAO-22-105133 Nursing Home Infection Control
As previously described, the Centers for Medicare & Medicaid Services
(CMS) monitors nursing home compliance with federal standards
primarily through the comprehensive standard surveys and as-needed
investigations state survey agencies conduct. Beginning in March 2020,
CMS required state survey agencies to conduct focused infection control
surveys, a new type of survey developed by CMS and the Centers for
Disease Control and Prevention (CDC) in response to the pandemic with
a narrower scope than a standard survey. Focused infection control
surveys assess federal standards for nursing home infection prevention
and control that could contribute to the transmission of COVID-19, such
as standards for personal protective equipment, testing, and isolating
positive cases. CMS also suspended standard surveys and low priority
investigations to limit surveyor time on site and focus state survey agency
resources on limiting the spread of COVID-19.
1
Initially, state survey
agencies conducted the focused infection control surveys in nursing
homes specifically identified by HHS, and, beginning in June 2020, state
survey agencies were required to conduct the focused infection control
surveys any time a nursing home experienced a new COVID-19
outbreak.
2
Beginning in August 2020, CMS indicated state survey
agencies should resume standard surveys as soon as they have the
resources to conduct the surveys but also required them to continue
conducting focused infection control surveys.
3
In January 2021 and again
in November 2021, CMS changed the requirement for when a focused
1
Under section 1135 of the Social Security Act, the Secretary of the Department of Health
and Human Services (HHS) may temporarily waive or modify certain federal health care
requirements, including those relating to standard surveys of nursing homes, when both a
public health emergency and a disaster or emergency have been declared. 42 U.S.C. §
1320b-5. This authority was triggered on March 13, 2020, when the President declared
the COVID-19 outbreak to be a national emergency under the National Emergencies Act
and a nationwide emergency under section 501(b) of the Robert T. Stafford Disaster
Relief and Emergency Assistance Act. The Secretary of HHS had previously declared
COVID-19 a public health emergency on January 31, 2020, retroactive to January 27,
2020. See Centers for Medicare & Medicaid Services, QSO-20-20-ALL (March 20, 2020).
2
CMS also required state survey agencies to conduct focused infection control surveys in
all the nursing homes in their states by July 31, 2020 and in 20 percent of nursing homes
in their states starting in fiscal year 2021. CMS also authorized states to expand certain
survey activities, including standard surveys and high-priority complaint surveys, at the
state’s discretion. See Centers for Medicare & Medicaid Services, QSO-20-31-ALL (June
1, 2020) and Centers for Medicare & Medicaid Services, QSO-20-20-ALL (March 20,
2020).
3
See Centers for Medicare & Medicaid Services, Enforcement Cases Held During the
Prioritization Period and Revised Survey Prioritization, QSO-20-35-ALL (Baltimore, Md.:
August 17, 2020).
Appendix III: Types of Surveys and
Investigations to Assess Whether Nursing
Homes Are Meeting Federal Standards

Appendix III: Types of Surveys and
Investigations to Assess Whether Nursing
Homes Are Meeting Federal Standards
Page 41 GAO-22-105133 Nursing Home Infection Control
infection control survey must be conducted. Specifically, in November
2021, CMS required state survey agencies to perform focused infection
control surveys for 20 percent of nursing homes in their state annually,
prioritizing those facilities that report new COVID-19 cases and low
vaccination rates, in addition to continuing to conduct standard surveys
and investigations.
4
See figure 4 for a description of the types of surveys
and investigations used to assess whether nursing homes are meeting
federal standards as of April 2022.
4
On November 30, 2020, elements of the focused infection control survey were
incorporated into the standard survey process, in addition to maintaining the focused
infection control survey as a stand-alone tool. See Centers for Medicare & Medicaid
Services, QSO-20-31-ALL (January 4, 2021 revision). Also see Centers for Medicare &
Medicaid Services, QSO-22-02-ALL (Nov. 12, 2021).

Appendix III: Types of Surveys and
Investigations to Assess Whether Nursing
Homes Are Meeting Federal Standards
Page 42 GAO-22-105133 Nursing Home Infection Control
Figure 4: Types of Surveys and Investigations Used by State Survey Agencies to Assess Whether Nursing Homes Are
Meeting Federal Standards, as of April 2022

Appendix III: Types of Surveys and
Investigations to Assess Whether Nursing
Homes Are Meeting Federal Standards
Page 43 GAO-22-105133 Nursing Home Infection Control
a
Initially, state survey agencies conducted the focused infection control surveys in nursing homes
specifically identified by the Department of Health and Human Services, and beginning in June 2020,
state survey agencies were required to conduct the focused infection control surveys any time a
nursing home experienced a new COVID-19 outbreak. Beginning in August 2020, CMS indicated
state survey agencies should resume standard surveys as soon as they have the resources to
conduct the surveys but also required them to continue conducting focused infection control surveys.
In January 2021 and again in November 2021, CMS changed the requirement for when a focused
infection control survey must be conducted. Specifically, in November 2021, CMS required state
survey agencies to perform focused infection control surveys for 20 percent of nursing homes in their
state annually, prioritizing those facilities that report new COVID-19 cases and low vaccination rates,
in addition to continuing to conduct standard surveys and investigations.

Appendix IV: Number and Percentage of
Surveyed Nursing Homes with Infection
Prevention and Control (IPC) Deficiencies
Page 44 GAO-22-105133 Nursing Home Infection Control
Table 3: Number and Percentage of Surveyed Nursing Homes with Infection Prevention and Control (IPC) Deficiencies, by
Calendar Year and Deficiency Code
2018
2019
2020
2021
Deficiency codes that went into effect prior to the pandemic
F-880: IPC program
a
6,316 (43.3%)
6,283 (42.5%)
6,810 (44.2%)
5,265 (37.3%)
F-881: Antibiotic stewardship program
b
698 (4.8)
739 (5)
195 (1.3)
307 (2.2)
F-882: Infection preventionist role
c
n/a
n/a
138 (0.9)
240 (1.7)
F-883: Influenza and pneumococcal immunization
d
564 (3.9)
643 (4.4)
269 (1.7)
597 (4.2)
F-945: Infection control training
e
n/a
n/a
n/a
n/a
Deficiency codes that went into effect during the pandemic
F-884: Reporting to the National Healthcare Safety
Network
f
n/a
n/a
1,811 (11.8)
4,702 (33.3)
F-885: Reporting to residents, representatives, and
families
g
n/a
n/a
335 (2.2)
274 (1.9)
F-886: COVID-19 testing for residents and staff
h
n/a
n/a
424 (2.8)
576 (4.1)
F-887: COVID-19 immunizations
i
n/a
n/a
n/a
158 (1.1)
Total surveyed nursing homes
14,591
14,773
15,406
14,128
Source: GAO analysis of Centers for Medicare & Medicaid Services’ (CMS) data. | GAO-22-105133
a
F-880 is the deficiency code for not meeting federal standards for establishing and maintaining an
IPC program. This code went into effect as part of CMS’s restructuring of its deficiency codes on
November 28, 2017, replacing a prior deficiency code that had been in effect for several years.
b
F-881 is the deficiency code for not meeting federal standards for establishing an effective antibiotic
stewardship program. This deficiency code went into effect on November 28, 2017.
c
F-882 is the deficiency code for not meeting federal standards for designating an infection
preventionist. This deficiency code went into effect on November 28, 2019. State survey agencies
began surveying nursing homes on it beginning August 26, 2020.
d
F-883 is the deficiency code for not meeting federal standards for influenza and pneumococcal
immunizations. This deficiency code went into effect as part of CMS’s restructuring of its deficiency
codes on November 28, 2017, replacing a prior deficiency code that had been in effect for several
years.
e
F-945 is the deficiency code for not meeting federal standards for infection control training. This
deficiency code went into effect on November 28, 2019, but, at the time of our review, CMS had not
yet directed state survey agencies to begin surveying homes on it. In June 2022, CMS announced
that state survey agencies should begin surveying homes on this deficiency code beginning October
2022.
f
F-884 is a COVID-19-specific deficiency code for not meeting federal standards for weekly COVID-19
reporting to the National Healthcare Safety Network. This deficiency code went into effect on May 6,
2020. Review for F-884 is conducted off-site by federal surveyors, who automatically cite nursing
homes for not submitting timely and complete data of all reporting elements.
g
F-885 is a COVID-19-specific deficiency code for not meeting federal standards for reporting COVID-
19 cases to residents, representatives, and family. This deficiency code went into effect on May 6,
2020.
h
F-886 is a COVID-19-specific deficiency code for not meeting federal standards for COVID-19
testing for residents and staff. This deficiency code went into effect on August 26, 2020.
i
F-887 is a COVID-19 specific deficiency code for not meeting federal standards for COVID-19
immunizations. This deficiency code went into effect on May 11, 2021.
Appendix IV: Number and Percentage of
Surveyed Nursing Homes with Infection
Prevention and Control (IPC) Deficiencies

Appendix V: Federal Nursing Home Infection
Prevention and Control (IPC) Actions
Page 45 GAO-22-105133 Nursing Home Infection Control
Table 4: Nursing Home Infection Prevention and Control (IPC) Actions Taken by the Centers for Medicare & Medicaid Services
(CMS) and the Centers for Disease Control and Prevention (CDC)
Date
Action
Actions prior to the pandemic
2009
CDC helped develop the National Action Plan to Prevent Healthcare-Associated Infections: Roadmap to
Elimination, a plan intended to coordinate and maximize the efficiency of healthcare-associated infection prevention
efforts across the federal government.
a
CDC began working with state-based Healthcare-Associated Infection programs, which are able to provide on-the-
ground IPC assessments and technical assistance in nursing homes and other health care facilities.
2012
CDC created a module in the National Healthcare Safety Network for national infection surveillance that allowed
nursing homes to voluntarily report infections, such as C. difficile.
b
CDC conducted the National Survey of Long-Term Care Providers to collect information from nursing homes on
IPC practices, as well as the immunization status of and infection burden among nursing home residents.
CMS begins to publicly report influenza and pneumococcal vaccination of nursing home residents and other IPC
quality measures, such as urinary tract infections among residents.
2015
CDC developed and released the Core Elements of Antibiotic Stewardship for Nursing Homes which outlines steps
nursing homes and other long-term care facilities could take to improve antibiotic prescribing practices and reduce
their inappropriate use.
2016
CDC developed and released an IPC assessment tool to assist health departments and facilities assess infection
control programs and practices in nursing homes and other long-term care facilities.
c
CMS published a final rule revising requirements for nursing homes’ broader IPC program with varying
implementation dates. The requirements implemented in 2016 included that nursing homes must have a system for
preventing, identifying, reporting, investigating, and controlling infections and communicable diseases for residents
and staff.
d
2017
CMS required nursing homes to develop an antibiotic stewardship program to combat the growing concern of multi-
drug resistant organisms.
e
2019
CMS and CDC collaborated on the development of a free on-line infection preventionist training course.
f
CMS and CDC released the Nursing Home Infection Control Worksheet, a nursing home self-assessment tool
developed through a 3-year pilot program across 40 participating nursing homes.
CMS required nursing homes to designate an infection preventionist who works at least part-time at the facility.
CMS updated surveyor interpretive guidance to clarify that a facility’s emergency preparedness planning should
include “emerging infectious diseases.”
Actions during the pandemic
Feb. 14, 2020
CMS created and released the “Head to Toe Toolkit,” offering educational materials and interventions for bedside
staff to prevent common infections.
Starting on Feb.
27, 2020
CDC conducted about 100 COVID-19 outbreak investigations in nursing homes and other long-term care facilities
in collaboration with local and state health departments.
g
Mar. 1, 2020
CDC issued guidance to assist nursing homes’ response to COVID-19.
h
CMS initiated blanket waivers to grant nursing homes flexibilities, such as waiving certain training and certification
requirements for certified nurse aides.
Mar. 4, 2020
CMS prioritized certain survey activities, such as surveys responding to allegations of abuse and neglect.
Mar. 13, 2020
CMS restricted all visitors and non-essential health care personnel from entering nursing homes, with exceptions
made for compassionate care situations, such as end-of-life situations. Surveyors were also granted access.
Cancelled communal dining and group activities.
Appendix V: Federal Nursing Home Infection
Prevention and Control (IPC) Actions

Appendix V: Federal Nursing Home Infection
Prevention and Control (IPC) Actions
Page 46 GAO-22-105133 Nursing Home Infection Control
Date
Action
Mar. 20, 2020
CMS temporarily suspended all standard surveys and suspended some other types of survey work.
CMS released a targeted IPC survey tool—the focused infection control survey—and instructed states to use this
survey in place of the standard survey process.
CMS suspended most enforcement actions for facilities not in substantial compliance, until revisit surveys could be
resumed.
May 6, 2020
CMS created new deficiency codes, known as F-tags (F-884 and F-885) associated with required reporting of
cases and deaths to CDC through the National Healthcare Safety Network and to residents, their representatives,
and their families.
June 1, 2020
CMS initiated a performance-based funding requirement tying CARES Act supplemental grants for state survey
agencies to the completion of focused infection control surveys.
CMS increased penalties for noncompliance with IPC, making the penalties more significant for those nursing
homes with a history of past IPC deficiencies or that caused actual harm to residents or immediate jeopardy.
CMS announced the deployment of Quality Improvement Organizations to provide technical assistance to
approximately 3,000 low-performing nursing homes that had a history of IPC challenges.
June 4, 2020
CMS announced it will post survey results that were conducted on or after March 4, 2020 on Nursing Home
Compare.
June 23 through
Aug. 19, 2020
CMS convened the Coronavirus Commission for Safety and Quality in Nursing Homes, a committee of experts
tasked to identify lessons learned from the early days of the pandemic and develop recommendations for future
actions to improve IPC measures in nursing homes.
i
July 18, 2020
The Department of Health and Human Services (HHS), including CDC and CMS staff, began sending strike teams
to nursing homes to assist with responding to COVID-19 outbreaks.
Aug. 17, 2020
CMS authorized the resumption of standard surveys.
Aug. 25, 2020
CMS released a national nursing home training program for frontline staff and management.
Aug. 26, 2020
CMS set civil monetary penalties for failure to report COVID-related data to the National Healthcare Safety
Network, associated with any F-884 citation.
Aug. 26, 2020
CMS created a new F-tag (F-886) associated with required COVID-19 testing of nursing homes staff and residents
and proper documentation of testing data.
CMS updated the focused infection control survey tool to assess compliance with new COVID-19 testing
requirements, as well as prior updates in guidance.
CMS temporarily updated the focused infection control survey tool to assess compliance with the requirement to
designate an infection preventionist.
Sept. 17, 2020
CMS changed restrictions on nursing home visitation to allow limited indoor visits while still adhering to social
distancing precautions.
j
Oct. 29, 2020
CDC launched the Project Firstline Healthcare Infection Control Training Collaborative, a coalition of health care,
public health, and academic partners who developed interactive infection control trainings for all health care
workers, including nursing home staff.
Nov. 30, 2020
CMS integrated elements of the focused infection control survey tool into the standard survey IPC pathway for all
standard surveys beginning after November 30, 2020, in addition to maintaining the focused infection control survey
as a stand-alone tool.
Dec. 4, 2020
CMS announced the agency will resume calculating nursing home health inspection and quality measure ratings on
January 27, 2021.
Dec. 21, 2020
CDC launched the federal Pharmacy Partnership for Long-term Care program to bring COVID-19 vaccine clinics to
residents and staff members in nursing homes across the country.
Jan. 4, 2021
CMS revised the criteria requiring states to conduct focused infection control surveys.

Appendix V: Federal Nursing Home Infection
Prevention and Control (IPC) Actions
Page 47 GAO-22-105133 Nursing Home Infection Control
Date
Action
Mar. 10, 2021
CMS further changed some visitation restrictions by allowing visitation even when a nursing home had COVID-19
positive residents and permitting physical contact between visitors and residents when a resident is vaccinated.
May 11, 2021
CMS published an interim final rule that established requirements regarding offering COVID-19 vaccines to
residents and staff and established an accompanying new F-tag (F-887). CMS also began requiring the reporting of
vaccination data to CDC.
k
Oct. 1, 2021
CDC, in partnership with CMS, provided funding for state-based strike teams to provide surge capacity, address
staffing shortages, and strengthen IPC activities in nursing homes.
l
Nov. 12, 2021
CMS announced steps to assist state survey agencies in addressing the backlog of complaint and standard
surveys. These steps included revising the criteria for conducting a focused infection control survey so that a survey
is not required in response to COVID-19 outbreaks and providing guidance for resuming standard surveys.
Nov. 12, 2021
CMS began allowing nursing home visitation for all residents at all times.
Dec. 28, 2021
CMS began issuing guidance requiring health care staff vaccination. Additional guidance was released in January
2022.
April 7, 2022
CMS announced plans to phase in an end to certain emergency declaration blanket waivers for nursing homes,
such as the waiver allowing nursing homes to employ nurse aides that have not completed a full course of training,
which would end on June 6, 2022.
m
Source: GAO summary of CMS and CDC identified actions. | GAO-22-105133
a
In 2013, the Department of Health and Human Services released an update to the National Action
Plan that included a chapter on healthcare-associated infection prevention across the long-term care
spectrum, including in nursing homes. Department of Health and Human Services, The National
Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination (Washington,
D.C.: 2013).
b
Between 2016 and 2018, CDC, CMS, and a network of health care quality improvement
organizations enrolled 2,000 nursing homes in the National Healthcare Safety Network to report and
track C. difficile bacterial infections in order to support antibiotic stewardship and infection prevention
practices. C. difficile is a bacterium that causes severe diarrhea and inflammation of the colon and
infections result in disproportionately higher rates of hospitalization and death in individuals over the
age of 65. During the pandemic, CDC expanded the National Health Care Safety Network to allow for
reporting of COVID-19 cases and deaths from nursing homes, as well as other COVID-19 related
data such as nursing home access to testing, personal protective supplies, and staff and resident
vaccinations.
c
The IPC assessment for nursing homes–known as the Infection Control Assessment and Response
tool–was later adapted and used by health departments and other partners to perform remote video-
assisted or onsite assessment of COVID-19-specific IPC practices and guide quality improvement
activities in nursing homes.
d
Medicare and Medicaid Programs; Reform of Requirements for Long-Term Care Facilities, 81 Fed.
Reg. 68,688 (Oct. 4, 2016).
e
CDC also implemented initiatives to address antibiotic-resistant infections and to promote antibiotic
stewardship efforts in nursing homes to align with CDC’s activities to address antibiotic resistance as
outlined in the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria, which was
issued in 2015. See The White House, U.S. National Action Plan for Combating Antibiotic-Resistant
Bacteria (Washington, D.C.: March 2015).
f
CMS also created a nursing home antibiotic stewardship program training.
g
CDC has provided further outbreak investigation and support services. For example, according to
CDC officials, the CDC funded Healthcare-Associated Infection and Antimicrobial Resistance
Prevention Programs that assisted with over 21,000 COVID-19 outbreak investigations in nursing
homes. CDC staff along with state and local health departments also conducted thousands of
Infection Control Assessment and Response assessments (both in-person and by telephone) in long-
term care facilities, including nursing homes.
h
Specifically, on March 1, 2020, CDC issued Responding to COVID-19 in Nursing Homes and
Performing Facility-wide SARS-CoV-2 Testing in Nursing Homes, as a supplemental addition to
Appendix V: Federal Nursing Home Infection
Prevention and Control (IPC) Actions
Page 48 GAO-22-105133 Nursing Home Infection Control
CDC’s overall IPC guidance, initially released January 28, 2020. The nursing home-specific guidance
was updated multiple times during the pandemic. In addition, on March 17, 2020, CDC began a
series of clinician outreach and communication activity calls on COVID-19 in nursing homes and
other long-term care facilities. See Centers for Disease Control and Prevention, Interim Infection
Prevention and Control Recommendations to Prevent SARS-CoV-2 Spread in Nursing Homes,
accessed on November 8, 2021,
https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html. This online guidance was
merged with Centers for Disease Control and Prevention, Responding to COVID-19 in Nursing
Homes and Centers for Disease Control and Prevention, Performing Facility-wide SARS-CoV-2
Testing in Nursing Homes, as of March 29, 2021.
i
The Commission’s final report was issued in September 2020. MITRE, Coronavirus Commission on
Safety and Quality in Nursing Homes, Commission Final Report (McLean, Va.: The MITRE
Corporation, 2020). This report was written for CMS under a government contract.
j
CMS also changed restrictions on communal activities and dining.
k
Medicare and Medicaid Programs; COVID-19 Vaccine Requirements for Long-Term Care Facilities
and Intermediate Care Facilities for Individuals with Intellectual Disabilities Residents, Clients, and
Staff, 86 Fed. Reg. 26,306, 26,336 (May 13, 2021).
l
The purpose of the funding is to assist nursing homes during their response to COVID-19 infections,
and to build and maintain the infection prevention infrastructure necessary to support resident, visitor,
and facility healthcare personnel safety. According to CDC officials, funding to health departments to
conduct these activities has been distributed and CDC continues to provide technical expertise and
assistance to the recipients.
m
CMS had previously waived the requirement that nursing homes may not employ anyone for longer
than four months unless they met the training and certification requirements under section 483.35(d)
of title 42 of the Code of Federal Regulations.

Appendix VI: Comments from the Department
of Health and Human Services
Page 49 GAO-22-105133 Nursing Home Infection Control
Appendix VI: Comments from the
Department of Health and Human Services

Appendix VI: Comments from the Department
of Health and Human Services
Page 50 GAO-22-105133 Nursing Home Infection Control

Appendix VI: Comments from the Department
of Health and Human Services
Page 51 GAO-22-105133 Nursing Home Infection Control

Appendix VI: Comments from the Department
of Health and Human Services
Page 52 GAO-22-105133 Nursing Home Infection Control

Appendix VI: Comments from the Department
of Health and Human Services
Page 53 GAO-22-105133 Nursing Home Infection Control

Appendix VI: Comments from the Department
of Health and Human Services
Page 54 GAO-22-105133 Nursing Home Infection Control

Appendix VII: GAO Contact and Staff
Acknowledgments
Page 55 GAO-22-105133 Nursing Home Infection Control
In addition to the contact named above, Karin Wallestad (Assistant
Director), Sarah-Lynn McGrath (Analyst-in-Charge), Elise Pressma,
Kathryn Richter, Elaina Stephenson, and Julianne Flowers. Also
contributing were Isabella Guyott, Laurie Pachter, and Jennifer Whitworth.
Appendix VII: GAO Contact and Staff
Acknowledgments
GAO Contact
Staff
Acknowledgments
(105133)

The Government Accountability Office, the audit, evaluation, and investigative
arm of Congress, exists to support Congress in meeting its constitutional
responsibilities and to help improve the performance and accountability of the
federal government for the American people. GAO examines the use of public
funds; evaluates federal programs and policies; and provides analyses,
recommendations, and other assistance to help Congress make informed
oversight, policy, and funding decisions. GAO’s commitment to good government
is reflected in its core values of accountability, integrity, and reliability.
The fastest and easiest way to obtain copies of GAO documents at no cost is
through our website. Each weekday afternoon, GAO posts on its website newly
released reports, testimony, and correspondence. You can also subscribe to
GAO’s email updates to receive notification of newly posted products.
The price of each GAO publication reflects GAO’s actual cost of production and
distribution and depends on the number of pages in the publication and whether
the publication is printed in color or black and white. Pricing and ordering
information is posted on GAO’s website, https://www.gao.gov/ordering.htm.
Place orders by calling (202) 512-6000, toll free (866) 801-7077, or
TDD (202) 512-2537.
Orders may be paid for using American Express, Discover Card, MasterCard,
Visa, check, or money order. Call for additional information.
Connect with GAO on Facebook, Flickr, Twitter, and YouTube.
Subscribe to our RSS Feeds or Email Updates. Listen to our Podcasts.
Visit GAO on the web at https://www.gao.gov.
Contact FraudNet:
Website:
https://www.gao.gov/about/what-gao-does/fraudnet
Automated answering system: (800) 424-5454 or (202) 512-7700
A. Nicole Clowers, Managing Director, [email protected], (202) 512-4400, U.S.
Government Accountability Office, 441 G Street NW, Room 7125, Washington,
DC 20548
Chuck Young, Managing Director, young[email protected], (202) 512-4800
U.S. Government Accountability Office, 441 G Street NW, Room 7149
Washington, DC 20548
Stephen J. Sanford, Managing Director, [email protected], (202) 512-4707
U.S. Government Accountability Office, 441 G Street NW, Room 7814,
Washington, DC 20548
GAO’s Mission
Obtaining Copies of
GAO Reports and
Testimony
Order by Phone
Connect with GAO
To Report Fraud,
Waste, and Abuse in
Federal Programs
Congressional
Relations
Public Affairs
Strategic Planning and
External Liaison
Please Print on Recycled Paper.
