
Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=bfsn20
Download by: [Texas A&M University Libraries] Date: 12 January 2016, At: 11:28
Critical Reviews in Food Science and Nutrition
ISSN: 1040-8398 (Print) 1549-7852 (Online) Journal homepage: http://www.tandfonline.com/loi/bfsn20
Fish Protein Hydrolysates: Production,
Biochemical, and Functional Properties
Hordur G. Kristinsson & Barbara A. Rasco
To cite this article: Hordur G. Kristinsson & Barbara A. Rasco (2000) Fish Protein Hydrolysates:
Production, Biochemical, and Functional Properties, Critical Reviews in Food Science and
Nutrition, 40:1, 43-81, DOI: 10.1080/10408690091189266
To link to this article: http://dx.doi.org/10.1080/10408690091189266
Published online: 03 Jun 2010.
Submit your article to this journal
Article views: 1946
View related articles
Citing articles: 262 View citing articles

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
43
Critical Reviews in Food Science and Nutrition, 40(1):43–81 (2000)
I. INTRODUCTION
At this time there are huge amounts of pro-
tein-rich byproduct materials from seafood pro-
cessing plants discarded without any attempt of
recovery. At the same time many processors are
no longer allowed to discard their offal directly
into the ocean, resulting in a very high cost of
refining the material before it is discarded. To
meet the need of the seafood processing industry,
an alternative to discarding these byproducts
should be developed. Recovery and alteration of
the fish muscle proteins present in the byproduct
material and using these as functional ingredients
in food systems is a very exciting and promising
alternative. However, for the industry to develop
processes for byproduct recovery and utilization
it has to be more economically feasible than dis-
carding the byproducts.
Every year over 91 million tons of fish are
harvested, of which 29.5% is transformed into
fishmeal.
1,2
Possibly more than 50% of the re-
maining fish tissue is considered to be processing
waste and not used as food.
3
With a dramatically
increasing world population and a world catch of
fish presently on the verge of exceeding the esti-
mated sustainable limits of the suggested 100
million tons/year, there is obviously an increased
need to utilize our sea resources with more intel-
ligence and foresight. By applying enzyme tech-
nology for protein recovery in fish processing, it
may be possible to produce a broad spectrum of
Fish Protein Hydrolysates:
Production, Biochemical, and Functional
Properties
Hordur G. Kristinsson* and Barbara A. Rasco**
Institute for Food Science and Technology, The School of Fisheries, University of Washington, Seattle,
Washington 98105
Referee: Dr. George M. Pigott, President, Sea Resources Engineering Inc., 4525 105 Avenue, N.W., Kirkland, WA, 98033
* Corresponding author: Present address: Department of Food Science, University of Massachusetts at Amherst, Marine Foods
Laboratory, Marine Station, Gloucester, Massachusetts 01930; Fax: (978) 281-2618; E-mail: [email protected]
** Present address: Department of Food Science and Human Nutrition, Washington State University, P.O. Box 646376, Pullman,
Washington 99164
ABSTRACT: Considerable amounts of fish processing byproducts are discarded each year. By developing
enzyme technologies for protein recovery and modification, production of a broad spectrum of food ingredients
and industrial products may be possible. Hydrolyzed vegetable and milk proteins are widely used food ingredients.
There are few hydrolyzed fish protein foods with the exception of East Asian condiments and sauces. This review
describes various manufacturing techniques for fish protein hydrolysates using acid, base, endogenous enzymes,
and added bacterial or digestive proteases. The chemical and biochemical characteristics of hydrolyzed fish
proteins are discussed. In addition, functional properties of fish protein hydrolysates are described, including
solubility, water-holding capacity, emulsification, and foam-forming ability. Possible applications of fish protein
hydrolysates in food systems are provided, and comparison with other food protein hydrolysates where pertinent.
KEY WORDS: fish protein hydrolysates, fish protein, functional properties, chemical hydrolysis of fish protein,
enzymatic hydrolysis of fish protein, protein functionality, fish byproducts.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
44
food ingredients or industrial products for a wide
range of applications. This would utilize both
fishery byproducts or secondary raw materials
and, in addition, underutilized species that would
otherwise be discarded.
Enzymatic modification of proteins using
selected proteolytic enzyme preparations to cleave
specific peptide bonds is widely used in the food
industry.
4
Hydrolysis of food proteins has a long
history, mainly for vegetable and milk proteins;
these proteins are widely used in the food indus-
try. Most work on the hydrolysis of fish proteins
was conducted in the 1960s. Some fish protein
hydrolysate (FPH) preparations at that time were
quite successful.
5
During the 1960s, research was
directed to the production of cheap nutritious pro-
tein sources for rapidly growing developing coun-
tries, or toward animal feed production, primarily
through production of fish protein concentrates
(FPC). Little work has been done recently on
FPH, but some research has been directed into the
potential of using powdered hydrolysates in food
formulations. Many studies have resulted in fish
protein hydrolysates with excellent functional
properties. However, taste defects, specifically
bitterness, and process economics are still major
limiting factors for FPH applications.
This review gives an overview of the differ-
ent techniques for production of fish protein hy-
drolysates, past and present research on their prop-
erties, and various methods to study the extent of
hydrolysis and product functionality.
II. THE BIOCHEMICAL
CHARACTERISTICS OF FISH MUSCLE
PROTEIN
In foods, a protein is traditionally categorized
as a fibrous or globular protein based on its ter-
tiary conformation. Each type of food protein has
a unique molecular structure that determines its
functional properties, in addition to a range of
environmental conditions over which it exhibits
such properties.
6
These factors and their effect on
functionality are discussed in more detail later in
this review.
The functional and structural properties of
food proteins thus vary tremendously, and to fully
understand the process of protein hydrolysis it is
crucial to have a good understanding of the nature
of the protein substrate and the hydrolyzing agent.
During protein hydrolysate manufacture, the pro-
tein substrate is hydrolyzed by either a proteolytic
enzyme or an acid or base.
Our diet contains a wide variety of proteins
from different sources. It is generally accepted
that the relative concentration of dietary essential
amino acids is the major factor determining the
nutritional value of food protein.
7
Proteins de-
rived from animal sources are considered to be
nutritionally superior to those from plants be-
cause they contain a better balance of the dietary
essential amino acids. Of these egg and milk pro-
teins (casein) are frequently used as reference
proteins for evaluating protein quality. Proteins
derived from meat and poultry muscle are also of
very high quality and fish muscle proteins are
equally nutritious.
8
Fish muscle contains an ex-
cellent amino acid composition and is an excel-
lent source of nutritive and easily digestible pro-
teins.
9,10
However, because fish is extremely
perishable and because chemical composition can
vary, the utilization of fish as a basic raw food
material presents unique food processing prob-
lems.
11
The muscle of different animals is very simi-
lar, containing similar protein and similar amino
acid profiles. There are slight differences between
fish muscle and the muscle of land animals. These
are mainly associated with the differences in
muscle structure required for swimming and buoy-
ancy. Fish are supported by a mass of water, thus
the muscle fibers require less structural support
than those in land animals. Because of this, fish
muscle tends to have less connective tissue than
muscles from terrestrial animals, resulting in more
tender texture. Also, because of the unique move-
ment of fish, the structural arrangement of muscle
fibers is quite different from terrestrial animals. A
large fraction of commercially utilized fish stocks
are cold adapted or poikilothermic, and because
of this their muscle proteins have different bio-
chemical properties compared with those of en-
dothermic animals.
12
Poikilothermic characteris-
tics of fish proteins make them more heat sensitive
than mammalian muscle proteins, with a greater
tendency to denature at elevated temperatures.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
45
Fish muscle proteins from cold water species are
more prone to denaturation than those from tropi-
cal waters.
13,14
The T-50 values (temperature re-
quired for 50% denaturation) of fish muscle pro-
teins are also influenced by pH and were reported
to be 29 to 35°C at pH 7.0 and 11 to 27°C at pH
5.5.
15
Protein composition in muscles varies by
muscle type. Of the three types (striated, smooth,
and cardiac muscle) of muscles, the striated
muscles are the predominant form in fish. Striated
muscle tissue is arranged into muscle fibers that
are bound together by a connective tissue to make
a fiber bundle. Fish muscle has “white” and “dark”
meat.
15
The white meat is generally more abun-
dant, contains less lipids than the dark meat, and
is the most widely consumed type of muscle tis-
sue. It is composed of about 18 to 23% of protein,
depending on the species and time of harvesting.
Fish proteins can be divided further into different
groups based on their solubility. About 70 to 80%
of fish muscle are structural proteins. These struc-
tural protein are soluble in cold neutral salt solu-
tions of fairly high ionic strength. The remaining
20 to 30% contain sarcoplasmic proteins that are
soluble in water and dilute buffers, and a final
part of the structural protein, 2 to 3%, being in-
soluble connective tissue proteins.
11
Recent stud-
ies, however, challenge these generally accepted
solubility data, showing that the muscle protein
components can be highly soluble at low ionic
strenghts.
16,17
Myofibrillar proteins are the primary food
proteins of fish, comprising 66 to 77% of the total
protein in fish meat. The myofibril protein com-
plexes contain myosin and actin. These are the
main components of the thick filament, and thin
filament, respectively. Myosin comprises 50 to
60% of the myofibrillar contractile proteins, and
actin only 15 to 30%.
12,15
Myosin is the most
abundant of the single muscle proteins, making
up around 38% of the total, and is a large mol-
ecule containing two identical heavy chains
(223 kDa) and two light chains (22 and 18 kDa).
The molecule has two identical globular head
regions that incorporate the light chains and a
significant fraction of the heavy chains. The tails
of the heavy chains form very long α-helices that
wrap around each other
18
(Figure 1). Myosin can
be cleaved by proteases at two sites on the mol-
ecule, one recognized by both trypsin and chy-
motrypsin and the other by papain. Papain cleaves
near the head region, releasing the head from the
tail. Trypsin and chymotrypsin cleave further from
the head, dividing the molecule into two compo-
nents called the heavy meromyosin (with the head
region) and the light meromyosin, both with dif-
ferent functional properties.
Myosin molecules are connected via their head
region to the polymerized actin molecules in the
thin filaments due to the ATPase activity of the
head molecules. This complex is called actomyo-
sin and is responsible for muscle contraction and
relaxation. Actomyosin plays a major role in de-
termining the quality of fish meat because it is
quite labile and easily affected during processing
and storage. For example, during frozen storage
the actomyosin becomes progressively less soluble
and the flesh becomes increasingly tougher.
19
The thin filament is a complex of actin mol-
ecules making a double helix. Tropomyosin sits
within the grooves of the thin filaments and two
FIGURE 1. Fish myosine molecule.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
46
troponin molecules bind the actin filament at each
helical repeat. Actin is the most prominent pro-
tein of the three protein in the thin filaments,
making up about 13% of the total muscle pro-
teins. Actin occurs in two forms, G-actin, a spheri-
cal monomer, and F-actin, a large polymer that
connects to myosin. The thin filaments play a
very important role by regulating muscle contrac-
tion. From the point of view of muscle biochem-
istry, thin filaments are very important, however,
their content is low in meat and their role with
respect to food processing has not been studied
completely. Other contractile proteins of interest
are C-protein, α-, and β-actinin, connectin and
paramyosin; however, they are of limited interest
as food proteins. With respect to protein hydroly-
sis, the myofibrillar protein myosin, actin, or ac-
tomyosin are subject to enzymic cleavage and are
the greatest focus here.
III. PROTEIN HYDROLYSIS
Proteolytic modification of food proteins to
improve palatability and storage stability of the
available protein resources is an ancient technol-
ogy.
20
Hydrolysates can be defined as proteins
that are chemically or enzymatically broken down
into peptides of varying sizes.
21
Protein hydroly-
sates are produced for a wide variety of uses in
the food industry, including milk replacers, pro-
tein supplements, stabilizers in beverages and fla-
vor enhancers in confectionery products. The
benefits of hydrolyzing food proteins to make
functional protein ingredients and nutritional
supplements is a more recent technology, with the
first commercially available protein hydrolysates
appearing only around the late 1940s. Although
production is massive worldwide, the proper con-
trol of the process and the exact mechanism be-
hind protein hydrolysis is in most cases not fully
understood. Recent advances have given research-
ers insight into the connection between the pro-
cess/extent of hydrolysis and the physicochemi-
cal mechanisms responsible for specific functional
properties of the hydrolyzed protein. Recent re-
search on enzyme catalysis has also aided with
the proper selection of enzyme catalysts and pro-
cessing conditions to obtain better control over
the reaction and characteristics of the final prod-
uct.
Chemical and biological methods are the most
widely used for protein hydrolysis with chemical
hydrolysis used more commonly in industrial prac-
tices. Biological processes using added enzymes
are employed more frequently, and enzyme hy-
drolysis holds the most promise for the future
because it results in products of high functionality
and nutritive value. The chemical and biological
hydrolysis are discussed in more detail below,
with an emphasis on hydrolysis with added en-
zymes. In addition, there are many potential tech-
niques for extracting protein from animal tissue.
These include the use of aqueous and organic
solvents; the conventional processes of cooking,
pressing, drying, and hot oil extraction.
22
The
extraction of protein by means of solvent is also
worth mentioning due to its industrial and histori-
cal importance for fish protein recovery.
A. Chemical Methods for Protein
Hydrolysis
1. Chemical Extraction: The Making of
Fish Protein Concentrate
The extraction methods mentioned above,
other than the chemical and biological hydrolysis
methods, do not hydrolyze protein. They are used
primarily to concentrate intact protein by the re-
moval of water and oil from the substrate. The
method of solvent extraction has been frequently
employed when producing fish protein concen-
trate (FPC). The development of FPC was one of
the earliest attempts to recover fish protein from
processing waste and to produce a protein ingre-
dient from underutilized species. FPC was the
precursor to the field of enzyme hydrolysis of fish
proteins. A small but extensive research program
on the large-scale production of FPC by the Bu-
reau of Commercial Fisheries, now the National
Marine Fisheries Service (NMFS) of the Depart-
ment of the Commerce, began in 1961. The gen-
eral aim of the program was to study the manufac-
ture and use of FPC as a solution to global protein
malnutrition and as a potential economic stimulus
to the American fishing industry.
23
Solvent-ex-
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
47
tracted FPC (type A FPC) is produced by using
primarily isopropanol or azeotropic extraction with
ethylene dichloride, although other solvents such
as ethanol have been used successfully as well. A
standard process presented by Sikorski and
Naczk
24
shown in Figure 2 is to grind a whole or
eviscerated fish, extract it with isopropanol at a
low temperature (20 to 30°C) for 50 min, then
collect the supernatant and extract it twice again,
first at 75°C for 90 min in isopropanol and then at
75°C for 70 min with azeotropic isopropanol. The
final supernatant fraction is collected, dried, milled,
and screened to separate out bone particles. The
final product has a high biological value and is
colorless and odorless, with less than 1% lipids.
The problem with type A FPC is that it is not
readily soluble or dispersible in foods and has
poor emulsification properties.
9,25,26
Dubrow et al.
27
FIGURE 2. A production scheme for fish protein concentrate. (Adapted from Ref. 24.)
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
48
reported that FPC produced at higher tempera-
tures (50°C) compared with lower temperatures
(20°C) had significantly lower emulsifying prop-
erties, but both had very poor solubility. General
poor functionality, off-flavors the high cost of
production, and traces of solvent in the final prod-
uct made solvent extracted FPC commercially
unsuccessful despite concerted efforts.
3,28
Al-
though FPC lacks solubility, it reportedly has
good foaming properties over a wide range of pH
(pH 2 to 11), making strong, stable foams.
29–31
Despite problems with protein functionality, sol-
vent extraction is the method of choice for the
abundant fatty pelagic fish species such as sar-
dine, herring, and capelin because the protein is
effectively separated from the lipids, thereby re-
ducing stability problems normally associated with
residual oxidizable lipid. For fatty fish, isopro-
panol was a slightly more efficient solvent than
ethanol considering the residual amounts of lip-
ids, but absolute ethanol produced FPC of lighter
color and a neutral flavor.
32
Many studies with FPC have also been con-
ducted with solvent-extracted FPC as a substrate
for enzyme hydrolysis, both to defat the substrate
and to make it more accessible to enzymatic hy-
drolysis, with excellent functional and nutritional
properties.
5,25,33,34,35,36
However, enzymatic hy-
drolysis using FPC as a starting substrate resulted
in loss of some functional properties because of
excessive protein breakdown but increased nitro-
gen solubility.
30
Taste and odor problems are
generally minimized with a FPC starting mate-
rial.
33
Recent studies with solvent-extracted FPC
have produced FPC with better protein function-
ality. For example, recently Vareltzis et al.
37
stud-
ied the addition of ethanol-extracted FPC made
from sardine to hamburger patties and found that
the overall functional properties (water binding
and cooking yield) and the penetration depth and
shear force value of the hamburger increased with
the addition of FPC. However the hamburgers
had a slightly unfavorable fishy flavor. Hoyle and
Merritt
5
found that herring protein extracted with
ethanol in a similar manner and then hydrolyzed
with either Alcalase or papain produced a hy-
drolysate with a markedly reduced bitterness and
less fishy odor.
2. The Chemical Hydrolysis Process
Chemical hydrolysis of proteins is achieved
by cleaving peptide bonds with either acid or
base. Several processes have been proposed for
the acid or alkaline hydrolysis of fish.
33
This has
been the method of choice in the past for the
industry primarily because it is relatively inex-
pensive and quite simple to conduct. There are,
however, many limitations to food ingredients
using this method. Chemical hydrolysis tends to
be a difficult process to control and almost invari-
ably leads to products with variable chemical
composition and functional properties.
21,38
Pro-
tein hydrolysis with strong chemicals and sol-
vents is performed at extreme temperatures and
pH and generally yield products with reduced
nutritional qualities, poor functionality, and re-
stricted to use as flavor enhancers.
39,40
a. Acid Hydrolysis
Acid hydrolysis of proteins is used more com-
monly than hydrolysis under alkaline conditions.
A vast majority of hydrolyzed proteins consumed
in the U.S. are prepared by acid hydrolysis, mostly
from inexpensive vegetable protein sources that
otherwise would have poor nutritive and little
functional value in foods. Although the process is
harsh and hard to control, it is still the preferred
method for hydrolyzed vegetable proteins. Hy-
drolyzed vegetable protein, which are widely used
for flavor and taste enhancement properties, re-
quire extensive acid hydrolysis.
38
Applications of
hydrolyzed vegetable proteins are primarily as
flavoring agents in processed meat, crackers, and
soup mixes. Acid hydrolysis of fish protein has
usually involved reacting fish proteins with hy-
drochloric acid, or in some cases sulfuric acid,
and the proteins are completely hydrolyzed at
high temperature, and often high pressure. The
hydrolysate is then neutralized to pH 6.0 to 7.0
and concentrated to either a paste or further dried.
41
Because the product is hydrolyzed extensively,
its primary functional property is high solubility.
Total hydrolysis of fish protein substrate can be
achieved in 18 h at 118°C in 6N hydrochloric
acid.
42
In addition, following the neutralization of
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
49
the digest, the hydrolysate contains large amount
of salt (NaCl), which can make the product unpal-
atable and interferes with functionality in food
systems. Another drawback of acid hydrolysis is
the destruction of tryptophan, which is an essen-
tial amino acid. Orlova et al.
43
proposed an acid
hydrolysis process of whole fish, where steam
distillation is used to remove aromatic substances
followed by filtration then concentration. The
concentrate was used in dehydrated soup cubes
and as a microbial media.
43
The acid hydrolysis is
also widely utilized to convert underutilized and
secondary raw material from fish into fertilizer
due to the low production cost and resulting ex-
tensive hydrolysis.
b. Alkali Hydrolysis
The use of alkali reactants, primarily sodium
hydroxide, to hydrolyze protein often results in
poor functionality and more importantly can ad-
versely affect the nutritive value of the hydroly-
sate. Despite this, limited alkali treatment is used
in the food industry to recover and solubilize a
broad range of proteins. For example, mechani-
cally deboned turkey residue (MDTR) includes a
significant proportion of alkali-soluble proteins
that can be recovered by alkali treatment and used
in food applications. Fonkwe and Singh
44
dis-
cussed the use of alkali extraction to recover
MDTR with an alkaline sodium chloride solution
but found it to be unsuitable due to low recovery.
Alkaline hydrolysis of fish proteins has primarily
used FPC as the starting substrate. During alka-
line hydrolysis of fish protein, rapid cleavage to
large water-soluble polypeptides takes place, fol-
lowed by further degradation at a slower rate.
Alkali treatment can aid in modifying the proper-
ties of insoluble FPC.
24
Tannenbaum et al.
45,46
have studied the alkaline process for hydrolyzing
insoluble FPC and its applications. They devel-
oped a small-scale batch process that utilizes high
pH (12.5) and 95°C for 20 min. The product
consisted of large peptides, some relatively in-
soluble at the isoelectric point, but with an overall
improvement in functionality with respect to the
original FPC. Use of the solubilized FPC as a
milk substitute gave a product far superior to that
obtained with FPC starting material, which had
poor solubility and dispersibility.
Several deleterious reactions occur in alka-
line solutions during hydrolysis. These are initi-
ated by hydrogen abstraction from the alpha car-
bon of an amino acid and include racemization of
L-amino acids, which produces D-amino acids,
which are not absorbed by humans. Also, disul-
fide bonds are split with loss of cysteine, serine,
and threonine via β-elimination reactions and
formations of lysinoalanine, ornithinoalanine,
lanthionine, and β-amino alanine can also oc-
cur.
31
Some of these elimination and addition re-
actions may lead to the formation of toxic sub-
stances (e.g., lysinoalanine) that are highly
undesirable in foods.
47,48
Alkaline hydrolysis re-
action products have an inhibiting effect on pro-
teolytic enzymes, reducing the rate of hydroly-
sis.
49
Some of the possible reaction products that
may form during alkali hydrolysis are shown in
Figure 3.
24
High collagen solubility is also ob-
served with alkali treatment.
50
B. Biochemical Methods for Fish Protein
Hydrolysis
Biochemical hydrolysis to produce fish or
other food protein hydrolysates is performed by
utilizing enzymes to hydrolyze peptide bonds.
This can be done via proteolytic enzymes already
present in the fish viscera and muscle (endog-
enous proteases), or by adding enzymes from other
sources. To understand the process of enzymatic
hydrolysis, it is very important to understand the
nature and activity of proteolytic enzymes.
1. Proteolytic Enzymes
Enzymes are biochemical catalysts vital for
living beings, because they accelerate chemical
reactions between organic constituents within the
cells that otherwise would take an extremely long
time to complete. In food science and technology
enzymes are exploited to perform desired func-
tions in processing and analysis and to facilitate
the conversions of raw materials into higher-qual-
ity, more desirable foodstuffs.
51
Enzymes make
this possible because the active site of an enzyme
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
50
FIGURE 3. Possible chemicals that may form in the alkali-treated proteins.
is highly specific for certain substrates. Enzymes
catalyze only one specific reaction and function
by forming a complex with the substrate whose
transformation they catalyze.
Enzymes used by the food industry and in
food research are predominantly hydrolases, most
of which are carbohydrases, followed by pro-
teases and lipases. Proteases are among the best
characterized enzymes. Proteolytic enzyme prepa-
rations are economically the most important group
of enzymes, and their use is well established in
the food industry.
52
Proteases are categorized ac-
cording to the specificity of the peptide bonds
they attack (hydrolyze) and the mechanism by
which they act.
48
Four major classes of proteases
are known. They are designated by the principal
functional group in their active site: serine, thiol,
carboxyl and metallo.
53
Proteases are character-
ized further by their hydrolyzing mechanism into
endoproteinases or exopeptidases. The endo-
proteinases cleave/hydrolyze the peptide bonds
within protein molecules, usually at specific resi-
dues to produce relatively large peptides. The
exopeptidases systematically remove amino acids
from either the N terminus, called aminopepti-
dases, or the C terminus, called carboxypepti-
dases, by hydrolyzing the terminal peptide bonds.
In food protein hydrolysis, endoproteinases are
always used, but occasionally endoproteinases are
combined with exopeptidases to achieve a more
complete degradation.
20
Although the four classes of proteases men-
tioned above have different catalytic mechanisms,
they all share a common transition state (interme-
diate) during catalysis. To discuss enzyme kinet-
ics in any detail would require a separate review,
and this is not intended here. However, it is im-
portant to know the basic steps in enzyme cataly-
sis and to understand the mechanism of protein
hydrolysis. Some proteases preferentially cata-
lyze the hydrolysis of bonds adjacent to a particu-
lar amino acid residue, while others are less spe-
cific. The catalysis by proteases occurs primarily
as three consecutive reactions: (1) the formation
of the Michaelis complex between the original
peptide chain and the enzyme, (2) cleavage of the
peptide bond to liberate one of the two peptides,
and (3) a nucleophilic attack on the remains of the
complex to split off the other peptide and to re-
constitute the free enzyme.
20,54
The hydrolysis of
peptide bonds leads to an increase in the numbers
of ionizable groups (NH
3
+
and COO
-
), with a con-
comitant increase in hydrophobicity and net
charge, a decrease in molecular size of the polypep-
tide chain, and an alteration of the molecular struc-
ture leading to the exposure of the buried hydro-
phobic interior to the aqueous environment.
55–58
Giving the substrate the symbol S, the enzyme E
and the peptides in the reaction P, the overall
mechanism can be presented as:
E S ES EP H P
EOHPHP
k
k
k
k
HO
+←→→+−
′
→+−+−
′
1
1
2
3
2
–
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
51
This enzyme-substrate complex may dissoci-
ate back to reactant substrate and free enzyme, or
to free enzyme and product molecules. In other
words, classic Michaelis-Menten kinetics apply.
20
The generally accepted mechanism for proteases
indicates that the second step is the rate-determin-
ing step, thus k
2
primarily determines the overall
reaction speed, and K
m
is more or less equal to the
true dissociation constant. This simple mecha-
nism does not, however, deal with the detailed
question of how the enzyme and substrate are
bound or what molecular configurations lead to
product formation. To fully understand the ca-
talysis, a fairly detailed explanation is in order,
which is not the purpose of this review.
Enzymatic hydrolysis of proteins is a complex
process because of several peptide bonds and their
specific accessibility to enzymatic reactions.
47
The
specificity of enzymes is not the only factor that
affects the peptide profile of the final product.
Environmental factors such as temperature and pH
play an important role. Both temperature and pH
can greatly affect the enzyme reaction kinetics, and
the effect of these factors is different for each
enzyme. Generally, there is an optimum combina-
tion of both pH and T, where an enzyme is the most
active. Temperature and pH extremes deactivate
the enzymes by denaturing them.
2. Autolytic Hydrolysis
Biochemical production of fish protein hy-
drolysates may be carried out by employing an
autolytic process. An autolytic process depends
on the action of the digestive enzymes of the fish
itself. There are no enzyme costs involved, and it
is a simple operation.
59
The end product of au-
tolytic hydrolysis is generally a fairly viscous
liquid rich in free amino acids and small peptides.
The digestive enzymes in question are primarily
the serine proteases trypsin and chymotrypsin,
and the thiol protease pepsin, all major enzymes
of fish viscera and digestive tract. Lysosomal
proteases, or catheptic enzymes, present in fish
muscle cells also contribute to proteolytic break-
down to some extent.
The endogenous enzymes in autolytic hydroly-
sis are a very complex mixture of enzymes, all
with different activity requirements, which result
in end products of different molecular profiles.
Another complication is that the presence of cer-
tain digestive enzymes and their concentration
may be highly seasonal, gender and age specific,
and can vary tremendously within a species as
well as between species. These variations make it
very hard to control the hydrolytic process, and
direct the production of hydrolysates with spe-
cific molecular properties. Autolytic methods such
as chemical methods often result in a final prod-
uct with bad functionality. Despite these prob-
lems, endogenous proteolytic enzymes are used
to produce hydrolyzed products, specifically fish
sauces and fish silage.
The production of fish sauce preceded fish
silage production and is the major fermented fish
product presently consumed in the world. Its pro-
duction has thousands of years of tradition in
Asia, and it is also known to have been produced
in Mediterranean countries in ancient times. Pres-
ently, fish sauce is used mainly as a condiment on
rice dishes like the popular Nuoc-Nam produced
in Vietnam, and the annual production in South-
east Asia is about 250,000 metric tons.
60
The
production of fish sauce does not require elabo-
rate processing equipment. The substrate is usu-
ally fish from one or more pelagic species, such
as anchovies or sardines, or minced whole fish of
low commercial value. The substrate is immersed
in a solution containing high concentrations of
salt (20 to 40%) and at relatively high tempera-
tures, preferably ambient tropical temperatures.
In the case of whole fish, the visceral proteolytic
enzymes start by hydrolyzing the stomach con-
tents, then work their way through the stomach
wall, and finally reach the muscle tissue, where
they join with catheptic enzymes to hydrolyze
fish muscle proteins. The pH of the fish sauce
process is usually neutral, because no base or acid
is added to the reaction mixture. The acid-depen-
dent proteolytic enzymes, such as pepsin and the
catheptic enzyme, contribute little to fish sauce
production. The endogenous serine proteases very
slowly breakdown the fish muscle for 6 to 12
months (Nuoc-Mam) under anaerobic conditions.
This slow but extensive breakdown results in a
liquefied fish sauce composed predominantly of
free amino acids, with up to 50% nitrogen recov-
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
52
ery. The high concentration of salt is responsible
for the slow hydrolysis because this slows the
activity of the proteolytic enzymes. More impor-
tantly, the high salt concentration and anaerobio-
sis totally inhibits growth of spoilage microor-
ganisms once the salt has fully penetrated the
tissue.
61
Lower concentrations of salt, however,
result in sauces with higher yield, lower levels of
volatile acids, and better balanced composition of
amino acids.
62
During autolysis to produce fish
sauce, a lipid phase, an aqueous soluble phase
that contains much protein but little lipid, and
insoluble sediment of protein and lipid is formed.
63
After the hydrolysis is completed, the liquid pro-
tein hydrolysate is tapped, filtered, and bottled,
with the final product containing up to 10% free
amino acids and low-molecular-weight peptides,
and 25% salt.
26,60
Although the production of fish
sauce does not improve the nutritive value of the
protein, the keeping quality is greatly increased
and organoleptic characteristics are generally
improved.
64
The process of manufacturing fish silage is
different, but the product shares many character-
istics with fish sauce. The production of fish si-
lage was not started until the middle of this cen-
tury, and it is far from being as widely employed
as fish sauce production. The application of fish
silage is primarily for animal feed production
instead of food applications. The process is rapid
and enzymes involved are very effective in pro-
ducing oil and proteins fractions that are readily
separated. The substrate is usually secondary raw
material from fish processing or underutilized fish
species. The substrate is mixed with strong min-
eral acids or organic acids such as formic acid to
acidify the mixture below pH 4. At this pH the
serine proteases are generally inactive, but pepsin
and the catheptic enzymes are highly active. The
pepsin content can be very high in the visceral
portion of fish, and the enzyme is primarily re-
sponsible for fish silage production. Under acidic
conditions the active endogenous enzymes par-
tially hydrolyze the fish over several weeks to
produce a slurry containing up to 12% amino
acids and peptides. Usually about 80% of the
protein in acid fish silage become solubilized af-
ter 1 week at temperatures around 23 to 30°C.
65
The rate is primarily dependent on conditions
such as ambient temperature and relative amounts
of the visceral organs present. The processing
time is much shorter than for fish sauce because
no salt is added.
26,60
The production of fish silage
using lactic acid bacteria as the hydrolyzing agent
has been reported.
66,67
The bacterial fermentation
is initiated by mixing minced or chopped fish
with a fermentable sugar that favors the growth of
lactic acid bacteria, which is advantageous be-
cause the bacteria produces acid and antimicro-
bial factors that inhibit competing bacteria.
65
The feed applications of fish silage are prima-
rily limited to young animals due to the extensive
hydrolysis of the proteins. For fish silage to be
incorporated successfully in animal feeds, it has
to contain the majority of the nitrogen fraction as
intact proteins or peptides rather than as free amino
acids, which are less well absorbed. A shorter
processing time and added commercial proteases
may be useful in such instances. Also, problems
connected with the development of bitterness in
the hydrolyzed silage can make the product highly
unpalatable not only for humans but also animals
fed feeds rich in fish silage. The utilization of fish
silage as a primary protein source in fish feeds has
been neither fully investigated nor commercially
successful; however, it is incorporated into diets
for pigs, poultry, and mink.
67
Research on fish hydrolysates made with en-
dogenous enzymes for human food applications
has been very limited. Fish sauce is almost the
only autolytically produced food of aquatic ori-
gin. The main reason for this is fairly straightfor-
ward. To produce a functional protein hydroly-
sate with specific properties, a good knowledge
of the enzymes involved is crucial. Endogenous
enzymes in fish are a complex and highly variable
mixture, and thus the properties of functional pro-
tein hydrolysates so prepared may vary greatly
under the same reaction conditions. Also, in the
U.S. the sale of processed fish foods containing
visceral material of any kind is prohibited by the
FDA. Despite this, some work on developing fish
silage for human consumption has been conducted.
In 1972, Malcolm B. Hale and a group of scien-
tists at NMFS and the University of Maryland
conducted a comprehensive study on making func-
tional FPH by enzymatic hydrolysis for human
consumption.
33
This study involved autolysis of
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
53
red hake (Urophycis chuss), a relatively lean fish,
and alewife (Alosa pseudoharengus), a very fatty
fish. The autolysis of raw hake was conducted at
optimal conditions for the native enzymes, 50°C
and pH 7.0, for 24 h where fish was 50% of the
total slurry. The resulting hydrolysate was either
spray dried or tested as a concentrate. The aver-
age yield of dry solids for hake was lower than for
any other proteolytic enzyme employed, 10.0%
(dry solubles/wet fish), compared with the high of
14.3% from using Alcalase. Similarly, the chemi-
cal score attained for soluble hydrolysate were
quite low due to low tryptophan content that is
correlated with a low recovery. The protein effi-
ciency ratios for both red hake and alewife hy-
drolysates were essentially equivalent to that of
casein. Also, the inclusion of insoluble solids in
the final product resulted in very high fat content.
The results for lipid pressed alewife were similar.
However, by lowering the reaction period to 4 h
and raising the temperature to 55°C, a satisfactory
product with good nutritional value was prepared.
Both the red hake and alewife were only 50 to
70% soluble, requiring substantial additions of
commercial enzymes, at uneconomic levels, to
become fully soluble. The alewife hydrolysate
also suffered from very fishy taste; however, the
red hake hydrolysate had a less fishy taste and
odor. Food applications of the products obtained
by Hale and co-workers were limited and could
be used primarily as a protein supplement in cul-
tures where its taste would be acceptable and the
caloric value of the lipid desirable.
33
Shahidi et al.
59
hydrolyzed ground capelin
(Mallotus villosus) by endogenous enzymes and
found that it enhanced the overall extraction of
the fish protein at both acid and alkaline pH, as
both acid and alkaline proteases are present in
fish muscle and viscera. The protein recovery of
hydrolysates produced autolytically was, however,
considerably lower compared with commercial
enzymes, 22.9% compared with 70.6% with
Alcalase. A recent study by Cui
68
with chum
salmon (Oncorhynchus keta) mince and visceral
content showed a surprisingly extensive and rapid
hydrolysis at an acid pH and 37°C. The hydroly-
sate also showed a marked difference in the mo-
lecular weight distribution of peptides when com-
pared with a commercial pepsin hydrolysis. The
native acid enzymes resulted in a product with the
majority of peptides of lower molecular weight
than the pepsin hydrolysate under the same ex-
perimental conditions. This indicates that protein
hydrolysates can be obtained through autolysis
very efficiently at relatively mild temperatures.
The functional properties of the product were,
however, not investigated.
The main limitation of work performed on
autolytic hydrolysis of food proteins is the lack of
research on functional properties. Studies have
shown that protein recovery can be adequate and
that nutritional requirements are good, but infor-
mation on functional properties of the resulting
hydrolysate is very important to successfully evalu-
ate its use in formulated foods.
3. Enzymatic Hydrolysis of Fish Muscle
Proteins with Added Enzymes
Using added enzymes to hydrolyze food pro-
teins is a process of considerable importance used
to improve or modify the physicochemical, func-
tional, and sensory properties of the native pro-
tein without jeopardizing its nutritive value, and
often protein absorption is improved. These en-
zyme-based processes occur under mild condi-
tions over a series of stages and do not produce
hydrolytic degradation products via racemization
reactions observed with both acid and alkaline
hydrolysis.
69
The process of using added enzymes
instead of chemicals or endogenous enzymes of-
fers many advantages because it allows good con-
trol of the hydrolysis and thereby the properties of
the resulting products.
59
Processes can be designed
to take advantage of substrate specificity and the
relative reaction rates of different enzymes under
the reaction conditions employed. The physico-
chemical and functional properties of hydrolyzed
fish proteins are discussed separately in a later
section.
Enzymatic hydrolysis has been employed on
a variety of different proteins derived from live-
stock and poultry meat,
39,44,47,70,71
milk,
57,72–76
and
plants.
77,78
Hydrolysis of fish and other aquatic
foods is also being seen more frequently in the
literature. Several different aquatic protein sources
have been investigated for the production of func-
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
54
tional fish protein hydrolysates. These include
Rastelliger canagurta and Barbus carnaticus
both Indian tropical fishes,
79,80
hake (Urophycis
chuss),
10,25,33
shark (Isurus oxyrinchus),
81,82
sardine (Sardina pilchardus),
35,36,83,84
herring
(Clupus harengus),
5
crayfish,
85
lobster (Panulirus
spp.),
86
pollack (Theragra chalcogramma),
87
cape-
lin (Mallotus villosus),
59
dogfish (Squalus
acanthias),
88
chum salmon (Oncorhynchus keta),
68
Pacific whiting (Merluccius productus),
89
and
Atlantic salmon (Salmo salar).
90
It can readily be
seen from the list above that the majority of the
sources represent underutilized species or are
connected to utilization of processing wastes.
Enzymatic hydrolysis of fish protein has been
employed primarily as an alternative approach for
converting underutilized fish biomass, which is
commonly used in making feed or even fertilizer
into edible protein products.
15,88
More recently,
fish processing waste, or, more appropriately,
secondary raw material, has been connected to
FPH studies. In many cases this is due to strict
government waste regulations. Many processors
are no longer allowed to discard their offal di-
rectly to sea, resulting in a very high cost of
refining the material before discarding. Second-
ary raw material is the material remaining after
fillets are removed, and if viscera is included, this
can represent something on the order of 64% of
the weight of whitefish, the protein content of this
waste being about 10%.
3
Hydrolysis of fish pro-
tein with selected proteolytic enzymes provides
the possibility of controlling cleavage degree of
protein in the substrate. Using suitable enzyme/
substrate ratios and reaction times, this permits
the production of hydrolysates with different
molecular structures and different functional prop-
erties that could find applications in various food
formulations.
82
The hydrolytic process and reaction condi-
tions differ between different substrates and en-
zymes used and also depend on the properties
desired for the hydrolysate. Most of the described
processes are conducted under research condi-
tions and may have limited applications in the
industry. Commercial production of fish protein
hydrolysates is still limited on a worldwide basis,
but has reached a significant level in a few coun-
tries, including France, Japan, and Southeast
Asia.
60
Commercial batch protein hydrolysis has
several disadvantages such as (1) high cost of
using large quantities of enzymes, (2) difficulty in
controlling the extent of reaction that can result in
nonhomogenous products consisting of fractions
of varying molecular weight, (3) low yields, and
(4) the need to inactivate enzymes by pH or heat
treatment at the end of the reaction, which adds to
the processing costs.
91
Also, the enzymes em-
ployed in the process cannot be reused.
20
Figure 4
outlines a fairly typical process for producing fish
protein hydrolysates. Each step is given a detailed
discussion below.
a. The Substrate and Its Preparation
Lean species, or material derived from them,
is the substrate of choice for enzymatic hydroly-
sis as problems with lipid oxidation can be re-
duced. From an economic standpoint, however,
the abundant underutilized pelagic fish would be
preferred. The small pelagics comprise 23% of
the world’s catch,
92
of which only 42% is used as
human food. These are mostly fatty species such
as herring, sardines, anchovies, and mackerel, and
FPH prepared from them would contain high
amounts of lipid, which would require additional
treatments such as centrifugation to remove ex-
cess fat.
93
The fewer steps that are involved in the
production, the more economically viable the
operation becomes. If a whole fish is used, it is
eviscerated and washed, then ground in a meat
grinder, usually mixed with an equal amount of
water and homogenized in a blender until a vis-
cous homologous mixture is achieved. In some
instances a buffer solution is added to the minced
fish, for example, phosphate buffer
1,88
and boric
acid-NaOH buffer.
85
The presence of buffer salts
may affect the final properties of the hydroly-
sates. In a study of whey protein hydrolysis,
Kuehler and Stine
72
decided not to buffer the
solutions because of the influence buffer salts
might have on foaming or emulsifying properties.
Processes for fatty and lean species are differ-
ent. If the FPH contains more than 1% fish fat, the
fat must either be removed by solvent extraction
or stabilized by antioxidants
3,60
such as butylated
hydroxytoluene, butylated hydroxyanisol,
5
or
propylgallate.
10
A fish protein hydrolysate with
high lipid content may darken. The formation of
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
55
brown pigments may result from aldol condensa-
tion of carbonyls produced from lipid oxidation
after reaction with basic groups in proteins.
5
Re-
searchers have developed many means of mini-
mizing the lipid content in FPH. To obtain a
product of a lipid content not exceeding 0.5% by
weight, as established by the Protein Advisory
Groups of FAO for a fish protein hydrolysate
suitable for human consumption,
94
Quaglia and
Orban
36,84
defatted ground sardines by extraction
with isopropanol three times (solvent: substrate
ratio 1:1) at 46°C for 30 min, and then homog-
enized the mixture with water. Hoyle and Merritt
5
used an ethanol (90%) extraction directly on
minced herring at the fish/ethanol ratio of 1:2 at
70°C for 30 min, then mixed with equal volume
of water, hydrolyzed the mixture, then spray dried
it. Through this procedure the lipid content was
reduced to 0.9 from 4.0% of raw herring. Also
before placing the treated substrate in the reaction
vessel, chemical agents such as NaCl, sorbic acid,
or ethanol are occasionally added to the minced
fish to minimize bacterial degradation,
24
espe-
cially if reaction conditions are at neutral or alka-
line pH. However, adding NaCl can reduce the
rate of hydrolysis, increasing reaction time. Etha-
nol can also adversely affect the reaction process
in too high concentration by inhibiting protease
activity, although sorbic acid has not been found
to affect hydrolysis in concentrations up to 0.5%.
24
b. The Choice of Enzyme
The water mince mixture is added to a reac-
tion vessel where the hydrolysis takes place. Of-
FIGURE 4. A flow sheet for the enzymatic hydrolysis of fish protein to make fish protein hydrolysate
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
56
ten a flask, ranging from 0.5 to 3 L with a close-
fitting multisocket lid, has been used. The sockets
in the lid usually carry: a stirrer, driven by a
overhead variable speed motor to ensure adequate
mixing of the system, a thermometer to monitor
temperature, a pH electrode to monitor pH, and a
“pH-stat” device, where acid or base is added to
maintain a constant pH (Figure 5). The tempera-
ture of the reaction vessel is controlled. After the
required temperature is achieved, the pH of the
slurry is adjusted to the desired value. It is impor-
tant that the mixture is well mixed and consis-
tently stirred when the pH is added to allow for
uniform distribution of the added acid or base.
Processing temperature and pH is normally se-
lected to optimize the kinetics of the selected
enzyme or enzyme mixture.
48
A commercial pro-
tease is added in varying concentrations depend-
ing on the rate of hydrolysis needed. Given a
particular enzyme and a particular substrate, any
hydrolysis process involves at least five indepen-
dent variables. These are S (protein substrate con-
centration: %N × 6.25), E/S (enzyme-substrate
ratio in % or in activity units per kg N × 6.25), pH,
T (temperature), and t (time).
95
A wide variety of commercial enzymes exist
that have been used successfully to hydrolyze fish
and other food proteins. Proteolytic enzymes from
plants and microorganisms are most suitable to
prepare fish protein hydrolysates.
26
Enzymes used
to hydrolyze fish protein have at least one com-
mon characteristic: they have to be food grade,
and, if they are of microbial origin, the producing
organism has to be non-pathogenic.
96
The choice
of enzyme(s) is usually determined by a combina-
tion of efficacy and economics.
48
The screening for a suitable enzyme in a pro-
cess or experiment is very important if the prod-
uct is to have predetermined properties. The
screening process can be conducted in a variety of
ways, and there is no standard methodology for
this selection, leaving it primarily up to the indi-
vidual researcher what method is most appropri-
ate. Good examples of selection procedures are
found in studies by Hale,
97
Cheftel et al.,
25
Arzu
et al.,
98
Rebeca et al.,
1
Baek and Cadwallader,
85
and Kristinsson and Rasco.
99
In the comprehen-
sive study by Hale,
97
the relative activities of
more than 20 commercially available proteolytic
enzymes were measured for the hydrolysis of a
washed and freeze-dried fish protein substrate
from haddock. Preliminary tests at 1 h, 40°C, and
pH 7 resulted in the plant enzyme ficin to be most
active, but with papain, also a plant enzyme, hav-
ing a much higher relative ranking. When the
enzymes were tested at 24 h the picture changed,
60% digestion (the set limit) was achieved fastest
by Pronase, which exhibited the greatest activity
per unit weight. The enzymes pepsin, papain, and
pancreatin were most suitable if the lowest cost
per unit of proteolytic activity was to be followed.
These cost estimates are less valid today due to
the commercial availability of bacterial enzymes.
Although many would prefer to use acid pro-
teases so microbial growth could be more easily
limited, they usually yield a product with low
protein yield and too excessive hydrolysis for
food use.
39,84,86
Therefore, milder enzymes at neu-
FIGURE 5. A typical enzymatic hydrolysis reaction system in the laboratory. (Adapted from Ref. 20.)
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
57
tral and slightly alkaline conditions have been
used more frequently in recent years. In some
cases, a high level of solubilization is desired.
The acid enzyme pepsin has been most successful
in solubilizing fish protein. Liu and Pigott
100
pro-
duced a high-quality, fluffy, water-soluble fish
protein hydrolysate by pepsin hydrolysis of rock-
fish fillets. Tarky et al.
101
used pepsin at 37°C and
pH 2.0 to hydrolyze the entire fish waste resulting
from filleted English sole. The final product, after
ultrafiltration and spray drying, was a creamy
white, nonhygroscopic, water-soluble hydrolysate
with low lipid content but very poor nutritional
value.
Alcalase, an alkaline enzyme produced from
Bacillus licheniformis and developed by Novo
Nordisk (Bagsvaerd, Denmark) for the detergent
industry, has been proven repeatedly by many
researchers to be one of the best enzyme used to
prepare functional FPH and other protein hydroly-
sates.
20,36,59,84,88,89
Shahidi et al.
59
successfully used
Alcalase to optimize processing conditions to
produce capelin protein hydrolysates. Alcalase-
treated hydrolysates exhibited superior protein
recovery (70.6%) compared with the alkaline pro-
tease Neutrase and papain. Alcalase-treated hy-
drolysates also had the lowest lipid content (0.18%)
and excellent functional properties. Quaglia and
Orban
36
studied the same three enzymes at opti-
mal conditions on enzymatic solubilization of
sardine proteins. Hydrolysates produced using
Alcalase and papain were almost identical in ni-
trogen recovery, which increased with increasing
enzyme concentration (70% recovery at a en-
zyme/substrate ratio of 4%). Neutrase-treated
hydrolysates at the same ratio had only over 20%
nitrogen recovery. Hydrolysates from Alcalase
and papain also exhibited better functional prop-
erties and high nutritional value than those from
Neutrase. Improved nitrogen recovery of fish pro-
tein hydrolysates with increase of protease con-
centration has been reported elsewhere.
1,79
Alcalase
and Neutrase were studied further recently by
Benjakul and Morrissey
89
on Pacific whiting solid
waste at pH 9.5, 60°C and pH 7.0, 55°C, respec-
tively. Alcalase had a considerably higher activ-
ity than Neutrase and led to a more efficient hy-
drolysis. Optimum conditions for Alcalase were
20 Anson Units (AU)/kg, 1 h reaction time, and
waste:buffer ratio of 1:1 (w/v) at 60°C and pH
9.5. The resulting hydrolysate had a high protein
content with excellent nitrogen yield (up to 70%)
and an amino acid composition comparable to
fish muscle. Further, Alcalase was found to be
the most cost-effective enzyme out of five en-
zyme preparations tested to hydrolyzed salmon
muscle proteins.
99
Other new enzyme prepara-
tions have shown excellent potential for hydro-
lyzing fish proteins to make highly functional
FPH, including Flavourzyme 1000L (Novo
Nordisk, Bagsvaerd, Denmark), Corolase 7089
(Rohm Enzymes; Somerset, NJ) and Corolase
PN-L (Rohm Enzymes, Somerset, NJ).
99
In an extensive paper,
33
Hale reported the
effects of various processing conditions and com-
mercially available proteolytic enzymes on yield
and composition of water-soluble fish protein
hydrolysates. He concluded that the hydrolysis of
raw hake (Urophycis chuss) with Alcalase at pH
8.5 or above gave the best balance of essential
amino acids and a high yield of soluble product,
followed by pancreatin (a mixture of serine pro-
teases). One of the first studies on added enzyme
hydrolysis of fish protein was with papain, due to
its favorable properties of pH and temperature
optima for activity.
79,80
Two fish species were
used as substrate, one freshwater, Barbus
carnaticus, and the other marine, Rastrelliger
canagurta, and studied at 40 and 55°C, and pH 5
and 7. Total solids and nitrogen recovery for both
species was high, with pH 7 having the highest
total solids and nitrogen recovery (69.7% at 55°C
for freshwater species) compared with pH 5, pos-
sibly attributed to better hydration at pH 7.
To properly compare enzyme activity on the
same substrate, it is necessary to determine the
general proteolytic activity units at specific reac-
tion conditions. Unfortunately, very few research-
ers have done this, and most compare enzyme
activity on a weight basis of enzymes used in the
reaction mixture. Adding enzymes on the basis of
weight is meaningless if relative enzyme activity
is to be compared, because enzyme activity per
weight is different for each enzyme under experi-
mental conditions used. However, there exist few
studies that use and compare enzymes on the
basis of their proteolytic activity. Gonzalez-Tello
et al.
69,102
studied three proteases on whey protein
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
58
substrate by adding them to the system based on
Anson Units (AU). Unfortunately, they did not
use the three enzymes at the same AU. In addi-
tion, the activity units were obtained from the
manufacturer and not assayed by the researchers.
Also, reaction conditions used in the hydrolysis
experiments were different from the conditions
used in the enzyme assays, thus making the activ-
ity units very unreliable.
Benjakul and Morrissey
89
studied the hydroly-
sis of Neutrase and Alcalase on Pacific whiting
solid wastes by adding the enzymes to the system
based on AU units. This study has the same limi-
tation as the studies by Gonzalez-Tello et al.
69,102
by using reaction conditions different than those
used to assay the enzyme for proteolytic activity
and relying on enzyme units provided by the sup-
plier instead of assaying the enzymes themselves.
Other studies on fish protein hydrolysis add en-
zymes according to AU units and also suffer from
these same limitations.
59,82
A study by Beddows
and Ardeshir
103
is the most carefully conducted
study in the literature with respect to using stan-
dardized relative enzyme activity. They assayed
three proteases, bromelain, ficin, and papain, by
using BApNA (Benzoyl-Arg-para-Nitroanilide) to
obtain some indication of the relative proteolytic
activities of these enzymes. They then added the
enzymes to a system of minced Ikanbilis
(Stolephorus sp.), a tropical fish, at the same ac-
tivity units as based on their assay. The assay,
however, involved different reaction conditions
than the hydrolysis experiment, which limited its
reliability. Assaying enzymes with BApNA also
only estimates the trypsin activity of the enzyme
preparation, not their general proteolytic activity.
Using BapNA, therefore, gives a less accurate
and and probably underestimated value of en-
zyme activity. In a research conducted by
Kristinsson and Rasco,
99
where salmon muscle
proteins were hydrolyzed, the enzymes were as-
sayed under the same reaction conditions as they
were used in the hydrolysis experiment. A syn-
thetic protein substrate for proteolytic activity,
Azocoll, was used to obtain a uniform level of
proteolytic activity for all enzymes used. Azocoll
is an insoluble cowhide preparation consisting
largely of collagen. The method is based on dye
release from the insoluble substrate Azocoll when
a proteolytic enzyme cleaves the peptide linkages
in it. The rate at which the dye is released can be
used to quantitatively measure the amount of pro-
teolytic enzyme(s) activity working in a solution
by measuring the absorbance at 520 nm. The
enzymes were then used to hydrolyze the sub-
strate, all at the same activity unit according to the
Azocoll assay, thus comparing them at the same
activity level on the same substrate. No reports in
the literature have taken this approach; however,
the use of Azocoll in fish protein hydrolysis ex-
periments has been reported by Ferreira and
Hultin.
104
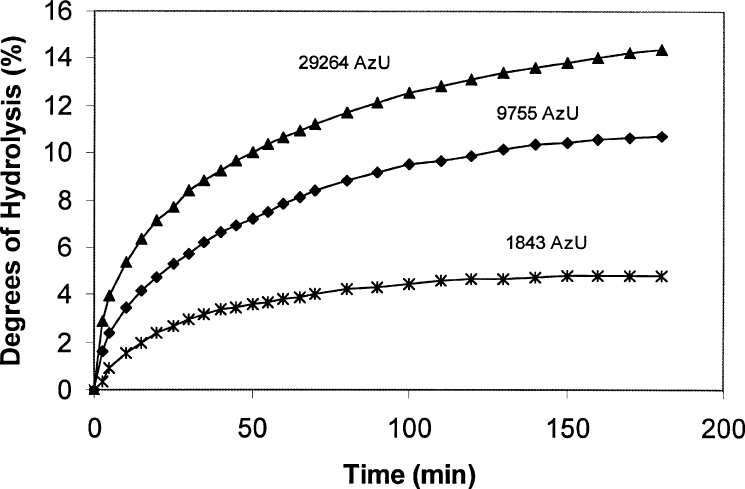
c. The Mechanism of Enzymatic Hydrolysis
The enzymatic hydrolysis of fish muscle pro-
teins is characterized by an initial rapid phase,
during which a large number of peptide bonds are
hydrolyzed, after this rate of enzymatic hydroly-
sis decreases and reaches a stationary phase where
no apparent hydrolysis takes place
59
(Figure 6).
The shape of the hydrolysis curve has been asso-
ciated with enzyme inactivation, product inhibi-
tion by hydrolysis products formed at high de-
grees of hydrolysis, a low K
m
value for the soluble
peptides that act as effective substrate competi-
tors to the unhydrolyzed fish protein,
1
and possi-
bly autodigestion of the enzyme.
105
Shahidi et
al.
59
found that a high concentration of soluble
fish peptides in the reaction mixture, released
during the initial phase of hydrolysis, reduced
both the rate of hydrolysis and the recovery of
soluble proteins. Thus, removal of hydrolysate
from the reaction mixture should enhance the
hydrolysis rate and the protein recovery.
By increasing the protease concentration, and
thereby increasing the extent of hydrolysis, re-
covery of soluble nitrogen increases,
1,71,103
al-
though increasing enzyme concentrations may not
be cost effective. Substrate concentration has also
negative effects on protein recovery. Linder et
al.
47
found that more than 8% protein concentra-
tion in the system, regardless of enzyme concen-
tration, seemed to have an inhibiting effect on
protein recovery. Baek and Cadwallader
85
reported
using Optimase to hydrolyze crayfish processing
byproducts the %DH increased as substrate con-
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
59
centration decreased to 45% (w/v), suggesting
that high %DH did not coincide with a high amount
of hydrolysate. Similarly, Surowka and Fik,
70
who
measured the production of protein hydrolysate
with Neutrase from chicken heads, reported that
hydrolysis increased as substrate concentration
decreased. Ferreira and Hultin,
104
using Newlase
A to hydrolyze cod (Gadus morhua) frames, found
that enzyme autolysis can be reduced at higher
substrate concentrations.
Because fish tissue is a very complex sub-
strate and also contains large amounts of protein-
ase inhibitors, it is impossible to explain the mecha-
nisms of protein hydrolysis in detail for this
system.
60
A kinetic study of the process is also
quite complicated due to the various types of
peptide bonds involved and their differing vulner-
ability to attack by enzymes during the hydrolytic
process.
69
Very few studies on kinetics of fish and
food protein enzymatic hydrolysis are reported.
Sakai et al.
87
conducted a kinetic study on the
hydrolysis of pollack surimi protein using a acid
protease derived from Aspergillus niger, deter-
mining the effects of temperature, pH, initial sub-
strate concentration, and enzyme concentration
on the kinetics of protein solubilization. Their
experimental data followed Michaelis-Menten
type kinetics. Michaelis-Menten kinetics has also
been observed with whey protein hydrolysis.
69
Kristinsson and Rasco
99
studied the kinetics of
five different enzyme preparations during the
hydrolysis of salmon muscle proteins and found
that the initial rate of the reaction for all enzymes
showed a linear relationship to enzyme activity.
These experiments indicated that the initial rate
constants for each enzyme tested were in the same
order. This confirmed previous studies by Hevia
et al.
106
and Cheftel et al.
25
on menhaden fish
protein as a substrate.
A kinetic model of whey protein hydrolysis
with Alcalase has been proposed, where the hy-
drolytic reaction is zero-order for substrate. The
enzyme denatures simultaneously via a second-
order reaction due to free enzymes attacking the
enzyme bound to the substrate.
69
Moreno and Cuadrado
107
hydrolyzed vegetable
proteins with Alcalase and found reaction mecha-
nism consistent with substrate inhibition and a
second-order deactivation with respect to the en-
zyme concentration. Enzyme autolysis was de-
FIGURE 6. Hydrolysis curve for salmon muscle mince with Corolase 7089 at three different activity units (AzU =
Azocoll Units).
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
60
pendent on the substrate concentration. Cheftel et
al.
25
used Pronase to hydrolyze fish protein con-
centrate and found that the rate constant decreased
with time, and that proteolysis did not follow
first-order kinetics with respect to concentration
of the peptide bonds. Cheftel et al.
25
suspected
this to be due to the multitude of possible sub-
strates in FPC, the number of different proteolytic
enzymes present, the inhibitory effects of sub-
strate or self-digestion, as well as the different
specificities that are known to be present in Pro-
nase. Archer et al.
108
also studied the kinetics of
the enzymatic hydrolysis of FPC. Their research
found that the enzyme is initially adsorbed to the
surface of the protein, with the initial rate of
reaction being proportional to the surface area of
substrate exposed to the aqueous phase. The over-
all kinetics were described by a sequence of two
first-order processes, an initial, fast reaction in
which loosely bound polypeptide chains are
cleaved from an insoluble protein particle, and a
second, slower reaction in which a more com-
pacted core protein is digested.
108
Langmyhr
109
studied the kinetics of the hydrolytic breakdown
of cod muscle with acetyltrypsin. The results in-
dicated that enzyme molecules are rapidly and
firmly bound to fish proteins and that the maxi-
mum binding occurred under the optimum pH
and T conditions for the enzyme. Results also
suggested that muscle proteins are hydrolyzed in
the same way and to the same extent whether they
occur separately or are integrated into intact
muscle.
d. Measuring the Extent of Enzymatic
Hydrolysis
To follow the reaction kinetics and get a
measure for the extent of the hydrolytic degrada-
tion, a parameter named degree of hydrolysis
(%DH) is employed. In principle, there are sev-
eral control methods, but under practical indus-
trial conditions there are few.
96
The degree of the
hydrolysis is most commonly used to describe
hydrolysis of food proteins. The advantage of
using %DH as a process parameter is that %DH
appears to determine unambiguously the proper-
ties of a protein hydrolysate for a given protein-
enzyme system.
96
The degree of hydrolysis dem-
onstrates both theoretically and empirically that
four processing variables, S, E/S, T, and t, can be
left uncontrolled, provided that %DH is con-
trolled.
95
From this, it is obvious that %DH is a
very simple and rapid method of measuring the
extent of protein breakdown.
Two methods for measuring %DH have been
studied thoroughly and shown to be satisfactory,
the pH-stat technique and osmometer technique.
The pH-stat method is more commonly used and
more useful for industrial applications. The prin-
ciple behind the pH-stat method is relatively simple
and is based on maintaining a constant pH during
the reaction. By pH-stat, the %DH is calculated
from the volume and molarity of base or acid used
to maintain a constant pH. The degree of hydroly-
sis is defined as the percent ratio of the numbers
of peptide bonds broken (h) to the total numbers
of bonds per unit weight (h
tot
; meq/kg protein,
calculated from the amino acid composition of
the substrate): %DH = (h/h
tot
) × 100.
%DH can also be expanded to:
%DH
BN
hMP
B
tot
=
⋅
⋅⋅
⋅
α
100
where B = base consumption in ml (or acid in
case of acid proteases), N
B
= normality of the base
(or acid), α = average degree of dissociation of
the –NH groups or COOH groups, MP = mass of
protein in grams (%N × 6.25).
The degree of
dissociation is found by the following equation:
α=
+
10
110
pH pK
pH pK
–
–
This equation for %DH is valid when hydrolysis
is conducted at a pH above the pK of the α-NH
group (for hydrolysis at neutral or alkaline pH).
Under these conditions, the reaction will result in
a net release of protons (H
+
) when peptide bonds
are cleaved and the base consumption is propor-
tional to the number of peptide bonds split.
110
Above pH 6.5, the dissociation of the protonated
α-NH group becomes significant. Therefore, it is
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
61
important to know the pK values of the α-NH
groups, both to determine the reaction pH and to
calculate the dissociation constant correctly. The
pK value varies significantly with temperature.
The pK values at different temperatures can be
calculated according to Steinhardt and Beychok:
111
pK
T
T
=+
⋅
⋅78
298
298
2400.
–
where T = temperature in Kelvin.
If samples are drawn during the experiment,
it is crucial to correct for the actual value of base
consumed. Actual base consumption (B′) will be
smaller than the theoretical base consumption (B)
if this is the procedure. To correct for this, B can
be calculated from the following formula:
20
∆∆TK C
f
=⋅⋅ω
where K
f
is 1.86 K/mol for water, ω the osmotic
coefficient, and C the osmolality of solution. From
the increase in osmolality, ∆C, %DH can be cal-
culated according to the following formula:
%
%
DH
C
Sf wh
osm tot
=
⋅
⋅⋅
⋅
∆ 11
100
where ∆C = depression of freezing point mea-
sured in milli-osmol, S% × f
osm
= g protein/1000
ml H
2
0, 1/w = calibration factor for the osmom-
eter: 1.04, h
tot
= total number of peptide bonds in
the substrate.
The f
osm
factor is calculated by knowing the
percent substrate dry matter, D%, present in the
reaction mixture:
f
D
osm
=
1000
100 – %
Although the osmometer technique is a very
good method to determine the degree of hydroly-
sis, it is being less employed frequently. The de-
gree of hydrolysis is now being more commonly
determined by using either the trichloroacetic acid
(TCA) method or the trinitrobenzenesulfonic acid
(TNBS) method. Both of these methods are very
useful when working within the pH 3 to 7 range,
where the pH-stat is unusable. Several variations
of these methods exist. The most commonly used
TCA method is based on determining the ap-
proximate degree of hydrolysis (%DH) of protein
hydrolysates by the ratio of percent 10% TCA
soluble nitrogen in the hydrolysate compared to
total amount of protein in sample. This is gener-
ally done by removing aliquots at selected inter-
vals and mixing with 20% TCA to create 10%
TCA-soluble and TCA-insoluble fractions. These
mixtures are then centrifuged and the supernatant
analyzed for nitrogen. The degree of hydrolysis is
thus calculated from the following equation:
%DH = (10% TCA-soluble N in sample/Total N
in sample) × 100
where B′
1
, B′
2
, etc. are the actual base consumption
at the drawing of samples number 1, 2, etc. The
value n is the sample number and m is the sample
size (ml).
The pH-stat method, however, has some
limitations. Use of pH-stat as a means of control
is only practical outside the approximate pH in-
terval 3 to 7.
96
This has to be taken into consider-
ation when deciding on pH values for the hy-
drolysis reaction. Within this pH range, process
control may depend on other methods. Therefore,
the pH-stat method for %DH determination is
useful mainly under alkaline conditions.
The other commonly used method to measure
hydrolysis, the osmometer technique, is more
universally applicable. This technique measures
the freezing point of samples drawn at regular
intervals during hydrolysis to construct a hydroly-
sis curve. For this method, the freezing point
depression (∆T) is measured and converted to
milli-osmol (∆C) as follows:
95
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
62
This method has been used successfully in fish
5
and other food protein hydrolysis.
44,70
The TNBS
method is based on the concentration of primary
amino groups in the hydrolysate. It is a spectro-
photometric assay of the chromophore formed by
the reaction of TNBS with liberated amino groups
(420 nm), at slightly alkaline conditions.
112
The
degree of hydrolysis can then be calculated as
presented by Baek and Cadwallader,
85
and
Benjakul and Morrissey,
89
for crayfish hydrolysis
and whiting hydrolysis, respectively:
%
–
–
max
DH
LL
LL
t
=⋅
0
0
100
where L
t
= the amount of a specific liberated
amino acid at time t, L
0
= the amount of the
specific amino acid in original substrate (blank),
L
max
= the maximum amount of the specific amino
acid in the substrate obtained after hydrolysis.
New methods and modifications of previous
methods for determining the degree of hydrolysis
are being developed. In the special case of fish, a
simple method for monitoring the enzymatic hy-
drolysis of fish protein was developed by Ukeda
et al.
113
The degree of hydrolysis was monitored
by an amino group determination method with
gluteraldehyde (GA). The method is based on the
consumption of dissolved oxygen during the re-
action between gluteraldehyde and the liberated
amino groups of the protein substrate. The results
by the GA method were in agreement with the
TNBS method previously described, with a corre-
lation coefficient of r = 0.992.
e. Termination of Enzymatic Reaction
When a desired %DH is attained, it is neces-
sary to terminate the enzymatic reaction. This is
very important as otherwise the enzymes would
remain active in the substrate and further hydro-
lyze the protein and peptides. Deactivation of
enzymes is achieved either by chemical or ther-
mal means. Usually the slurry of hydrolysate and
enzymes are transferred to a heat bath, where the
enzymes are deactivated by exposing them to
temperatures ranging from 75 to 100°C for 5 to
30 min, depending on the type of enzyme. For
example, papain is very heat tolerant, and has
been reported to need at least 90°C for 30 min to
be fully inactivated.
5
Terminating the reaction by
thermal means is undesirable
114
because of the
effects of heat denaturation on the protein that
leads to exposure of hydrophobic residues and
subsequently protein aggregation. Diniz and Mar-
tin
88
suggest that this form of heating can have the
advantage of being very effective in the separa-
tion of oil from the fish protein substrate, al-
though Webster et al.
39
found that some protein-
fat interaction occurred at elevated temperatures
that prevented their separation, when using bo-
vine lungs as the protein substrate.
The temperatures at which various proteins
denature and unfold vary enormously. Because
fish muscle proteins are adapted to function at
lower temperatures, they may not be particularly
heat stable. Denaturation is usually undesirable
because it results in altered physicochemical prop-
erties, particularly a loss in protein solubility and
functionality.
31
Spinelli et al.
34
are among few
researchers that have recognized the adverse ef-
fect elevated temperatures may have on fish pro-
tein hydrolysates. To both terminate the enzy-
matic reaction and quantitatively recover the
protein fractions, they reacted enzymatically hy-
drolyzed muscle proteins from rockfish with 5%
sodium hexametaphosphate (HP), following slight
acidification to form an insoluble protein-phos-
phate complex. By isoelectric precipitation, they
recovered up to 90% of the protein. In addition,
the complex could be washed free of occluded
nonprotein nitrogen components with no loss of
protein nitrogen.
Chemical inactivation would be to either lower
or raise the pH of the slurry to a point where the
enzyme deactivates. Some enzymes are more sen-
sitive to pH changes than they are to temperature
changes. Alcalase is a relatively thermostable
enzyme, but it is very sensitive to acid pH. Com-
plete inactivation of Alcalase therefore is obtained
by lowering the pH to 4.0.
20,59
Neutralizing the
slurry to pH 7.0 would inactivate pepsin and most
other acid proteases. Extremes of pH can also,
like elevated temperatures, have detrimental ef-
fects on protein and peptides. Many proteins un-
fold at pH values less than about 5 or greater than
10. Unfolding at such extremes of pH usually
occurs because the folded protein (or oligopeptide)
has groups buried in nonionized form that can
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
63
ionize only after unfolding.
52
Stabilizing salt-
bridges between ionizing groups can also be dis-
rupted by extreme pH values. The termination of
the enzymatic reaction thus is a formidable ob-
stacle in any FPH operation. The choice of inac-
tivation method should be carefully made and
based up on what the enzyme under study is
sensitive to, whether it is heat or pH. In some
cases a combination of elevating temperatures
and lowering pH have been used.
82
f. Protein Hydrolysate Concentration
Commonly, the slurry is desludged by cen-
trifugation, which results in several fractions (Fig-
ure 7): sludge in the bottom, aqueous layer in the
middle, lipid-protein fraction between aqueous
layer and sludge, aqueous and oil layers and oil
layer on the top.
68
A single centrifugation step can
eliminate the vast majority of the lipids present.
The oil layer overlying the aqueous layer is then
removed and the soluble fraction collected. Be-
cause lipid in the final hydrolysate is a major
concern for FPH, it is important to remove it.
Lipid residues in FPH must be lower than 0.5% to
prevent alteration of the lipid fraction during stor-
age.
34
More than one centrifugation step is often
required to separate the soluble proteins from the
lipids and insoluble solids. The second centrifu-
gation step would be performed only on the soluble
fraction. Other separation methods for fish pro-
tein hydrolysates have been reported such as suc-
tion filtration of the sludge
82
and filtering the
slurry by passing it through a 2-mm mesh screen.
86
The removal of colored and odiferous matter has
also been reported by treating the first soluble
fraction obtained by centrifugation with 1% w/v
charcoal at 55°C and 30 min before the next
separation step.
59
In a commercial operation, the final soluble
fraction is generally spray dried to convert the
hydrolysate to a powdered form, which can be
incorporated into food formulations. The insoluble
fraction or the sludge precipitated during cen-
trifugation may be used as animal feed. Spray
drying of the soluble fraction is one of the most
energy consuming and expensive steps in the pro-
duction of protein hydrolysates. In industrial pro-
duction a compromise must be made between the
hydrolysate yield and the amount of water that
must be removed to obtain a dry product.
60
A
suspension of equal amounts of water and fish
substrate appears to be convenient from an eco-
nomic point of view. Rebeca et al.
1
performed a
study where eviscerated mullet (Mugil cephalus)
was enzymatically hydrolyzed without the addi-
tion of water, to lower the cost of spray drying.
Interestingly, the experiment resulted in higher
dry matter content, with high protein recovery, of
the soluble fraction and the cost of drying was
reduced. Generally, in the laboratory, hydroly-
FIGURE 7. Different fractions obtained when recovering soluble fish protein hydroysates.
LIGHT LIPID-PROTEIN
AQUEOUS
SLUDGE
CLEAR OIL
LIPID-PROTEIN SMEAR
HEAVY LIPID-PROTEIN
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
64
sates are neutralized and freeze dried. By neutral-
ization the final product can have a fairly high salt
content, which is undesirable (HCl + NaOH →
H
2
O + NaCl). This can be mostly avoided if the
soluble fraction is passed through an ion exchange
column before freeze-drying. Dialysis to desalt
the soluble fraction can also be a useful process.
Increasingly, ultrafiltration membranes have
been introduced into the production process for
hydrolysates. The hydrolysate after enzyme inac-
tivation is filtered directly through a membrane
with a specific molecular weight cut-off value.
The ultrafiltration process can contain more than
one filter, yielding several molecular weight frac-
tions depending on the product desired. A major
advantage for such a process is the control of
molecular size of selected peptides so that a uni-
form product is possible,
76
as the molecular size
of the hydrolyzed proteins is a key factor in deter-
mining functional properties of hydrolysates.
115
The method has, however, not found its way into
fish protein hydrolysate production. The reason
probably being that in order to successfully use
the ultrafiltration, the sample has to be very pure
and free of lipids. It is, however, possible that the
method could be applied to highly purified and
defatted FPH powders. The method has found to
be very useful in soy,
115
whey,
75,116
and casein
protein hydrolysis.
76
In the case of whey protein,
large-molecular-weight fractions are primarily
responsible for allergic reactions. However, small
peptides and free amino acids are less well ab-
sorbed by humans. Therefore, protein hydrolysis
should not be more extensive than the minimum
required to eliminate allergic responses. By ultra-
filtration therefore it is possible to exclude both
the too large and too small peptides and collect
the molecular weight in between by passing the
hydrolysate solution through two filters, the first
with a higher cut-off value than the second.
116
Applying this technique to FPH would be an inter-
esting task to undertake.
IV. PHYSICOCHEMICAL AND
FUNCTIONAL PROPERTIES OF FISH
PROTEIN HYDROLYSATES
As previously mentioned, one of the major
advantages and goals of enzymatically hydrolyz-
ing fish proteins is to modify and improve their
functional properties. The functional properties
of fish protein hydrolysates are important, par-
ticularly if they are used as ingredients in food
products.
60
Enzymatic hydrolysis of fish proteins
generates a mixture of free amino acids, di-, tri-,
and oligopeptides, increases the number of polar
groups and the solubility of the hydrolysate, and
therefore modifies functional characteristics of
the proteins, improving their functional quality
and bioavailability. The choice of substrate and
proteases employed and the degree to which the
protein is hydrolyzed affect the physicochemical
properties of the resulting hydrolysates.
105
En-
zyme specificity is important to peptide function-
ality because it strongly influences the molecular
size and hydrophobicity of the hydrolysate.
117
Thus, the peptides obtained have different mo-
lecular profiles, and the surface energy of the
hydrolysate is different, depending on the en-
zyme used; these variations have a bearing on the
functionality of the mixture.
114,118
The more nar-
row the specificity, the are the larger peptides
produced; the broader the specificity, the smaller
are the peptides generated. As the range of enzy-
matic activities within commercial preparations
is increased, the hydrolysate becomes more com-
plex.
4
The chain length of peptides or breaking of
linkage is also dependent on the extent of hy-
drolysis; conditions of hydrolysis; concentration
of enzyme and the type of protein to be hydro-
lyzed.
41
Due to the complex peptide profile, it is
often useful to calculate the average peptide chain
length (PCL) introduced by Adler-Nissen and
Olsen
119
to express the composition of the protein
hydrolysate. PCL is calculated from the degree of
hydrolysis in the following way:
PCL
DH
=
100
%
This approximation is acceptable in nearly all
practical cases.
20
The relationship between PCL
and %DH is shown in Figure 8.
Manipulating the reaction conditions during
enzymatic hydrolysis of food proteins produces
hydrolysates with different solubility and emulsi-
fying characteristics, foaming properties, or taste
characteristics.
4
The control of the enzymatic re-
action is very important, as previously discussed.
Uncontrolled or prolonged hydrolysis of fish pro-
teins may result in the formation of highly soluble
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
65
peptides, completely lacking the functional prop-
erties of the native proteins. This may promote
the formation of undesirable bitter peptides. By
controlled hydrolysis it is possible to eliminate
these drawbacks and to obtain hydrolysates with
different physicochemical and functional proper-
ties, in some cases even better than the originals.
36
The physical and chemical properties that govern
protein functionality include size, shape, amino
acid composition and sequence, net charge and
distribution of charges, hydrophobicity/hydrophi-
licity ratio, peptide structures, molecular flexibil-
ity/rigidity, and the ability to interact/react with
other components.
120
Functionality of food pro-
teins has been defined as: “those functional and
chemical properties which affect the behavior of
proteins in food systems during processing, stor-
age, preparation and consumption”.
31
The follow-
ing sections discuss the main functional proper-
ties that fish protein hydrolysates exhibit, that is,
solubility, water holding, emulsifying, foaming
and sensory properties, and research aimed to-
ward evaluating these properties for various FPH
preparations.
A. Solubility
Solubility is probably the most important of
protein and protein hydrolysate functional prop-
erties. Many of the other functional properties,
such as emulsification and foaming, are affected
by solubility,
121
and therefore it is an excellent
indicator of the protein hydrolysate functionality,
and its potential (and limitations of) applica-
tions.
31,58
Hydrophobic and ionic interactions are
the major factors that influence the solubility char-
acteristics of proteins. Hydrophobic interactions
promote protein-protein interactions and result in
decreased solubility, whereas ionic interactions
promote protein-water interactions and result in
increased solubility. Ionic residues on the surface
of peptides and proteins introduce electrostatic
repulsion between protein molecules and repul-
sion between hydration shells around ionic groups,
both major contributors to increased solubility of
proteins. Solubility of protein and protein hy-
drolysates is generally measured by employing
the nitrogen solubility index (NSI), a standard-
ized method developed by AOCS and later modi-
fied by Morr et al.
122
The NSI is determined by
suspending a protein hydrolysate sample in water
then stirring and centrifuging the mixture. The
supernatant is then analyzed for nitrogen content
by the Kjeldahl procedure, and the NSI calculated
as a percentage of the soluble nitrogen to the
percentage of total nitrogen in the sample.
20
Intact fish myofibrillar proteins have the prob-
lem of the lack of solubility in water over a wide
range of pH,
13,123
and enzymatic hydrolysis is very
important in increasing the solubility of these
FIGURE 8. The relationship between average peptide chain length (PCL) and degrees of hydrolysis (%DH).
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
66
proteins. The effects of enzymatic hydrolysis on
fish protein are straightforward. Enzymatic break-
down of the protein involves a major structural
change in that the protein is gradually cleaved
into smaller peptide units, and as the degree of
enzymatic hydrolysis increases the solubility of
fish proteins increases. The enhanced solubility
of the hydrolysates is due to their smaller molecu-
lar size compared with the intact protein, and the
newly exposed ionizable amino and carboxyl
groups of the amino acids, that increase the hy-
drolysate hydrophilicity.
58,117
To effectively bind
to water molecules, the peptides have to have the
ability to form hydrogen bonds between its hy-
drophilic polar amino acid side groups and the
water molecules. Hydrolysis exposes some of the
hydrophobic groups to the surface, but at the
same time converts even more hydrophobic groups
to hydrophilic groups by generating two end car-
bonyl and amino groups. Thus, the smaller pep-
tides from myofibrillar protein hydrolysis have
proportionally more polar residues, with the in-
creased ability to form hydrogen bonds with wa-
ter. This increases protein solubility to that of the
intact protein. In addition, ion-dipole interactions
between water and simple ions such as Na
+
and
Cl
-
are also important in the interactions between
the polar or charged groups on biomolecules and
water.
18
Biomolecules tend to be very soluble at
favorable NaCl conditions. The increased solubil-
ity is partly through the formation of sodium salts
of carboxyl groups of proteins, COONa.
13
This is
true for fish myofibrillar proteins, but the hydro-
phobicity of globular proteins, such as milk pro-
teins, may increase with exposure of apolar amino
acid residues after hydrolysis.
117
Although in-
creased solubility has a positive relationship to
the extent of hydrolysis, care has to be taken that
the substrate is not too extensively hydrolyzed. A
very high degree of hydrolysis may lead to high
solubility, but this can have very negative effects
on the rest of the functional properties. To main-
tain or improve functionality, generally low de-
grees of hydrolysis are necessary.
4
Sugiyama et al.
84
studied enzymatic hydroly-
sis with several alkaline, neutral, and acid pro-
teases on defatted sardine meal. The result showed
that all the alkaline proteases had higher ability to
produce highly soluble protein hydrolysates com-
pared with the neutral and acid ones. Gel chroma-
tography showed that hydrolysates prepared with
the alkaline proteases had a lower average mo-
lecular weight. The increased hydrophilicity of
these preparations could be due to their larger
charge to size ratios compared with the longer
proteins such as those found in nonhydrolyzed
proteins from a similar source.
44
Interestingly,
pepsin had lower solubilized protein ratio than all
of the alkaline proteases, but pepsin is generally
considered one of the best enzymes to solubilize
fish protein.
35,36,68,97,100
Quaglia and Orban
35,36
also
studied the properties of hydrolysates produced
from sardine and concluded that Alcalase and
papain, at optimum pH and temperature, both
gave hydrolysates characterized by high solubil-
ity. Hydrolysates made with Alcalase at higher
degrees of hydrolysis showed a decrease in high-
molecular-weight fractions, and increased solu-
bility.
35
This connection between %DH and solu-
bility is also reported for other food protein.
124
A
significant increase in solubility was observed
with an increase in %DH from 2.5 to 15% for an
enzymatically hydrolyzed single cell microbial
protein (“Pruteen”), from less than 10% solubility
to over 90%.
124
Similar results were observed
with soy protein hydrolysates from 1 to 8.3%
DH.
96
Yu and Fazidah
125
hydrolyzed Aristichthys
noblis, a Chinese freshwater fish, with protease P
“Amano” 3, and reported excellent solubility at
15% DH after a 3-h digestion.
Hoyle and Merritt
5
found that enzymatically
hydrolyzed ethanol-extracted herring FPH had
highest solubility compared with nonextracted
enzymatically hydrolyzed FPH. This finding sug-
gests that the lower lipid content of ethanol-ex-
tracted herring FPH may have resulted in less
competitive water binding of proteins compared
with hydrolysates with higher lipid contents. Vieira
et al.
86
studied the functional properties of hy-
drolysates from lobster processing wastes and
found these to be highly soluble, pepsin yielding
a fraction with higher solubility than papain or a
fungal protease. The highest nitrogen solubility
index (NSI) for these hydrolysates was found at
extremes of pH. The solubilities of all the prod-
ucts were low at pH 5, approximately correspond-
ing to the isoelectric point of protein, at which it
precipitates. Shahidi et al.
59
also found that cape-
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
67
lin protein hydrolysates (CPH) prepared by dif-
ferent enzymes had different solubility profiles at
different pH conditions. However, in contrast to
the findings of Vieira et al.,
86
Shahidi et al.
59
found lower solubility at pH 7 to 8, and with close
to 100% solubility for Alcalase at pH 5. The
lowest solubility value at pH 7 to 8 was, however,
84%, thus CPH had excellent solubility over the
range of pH 2 to 11. Hydrolyzed salmon muscle
proteins exhibited excellent solubility at 5 to 15%
DH, between 92 to 100%, with over 96% solubil-
ity in the pH range of 2 to 11.
90
The solubility of enzymatically hydrolyzed
fish protein is generally much higher than for
chemically produced fish protein concentrate
(FPC). A FPC type-B air-dried fish powder had
extremely poor solubility at a wide range of NaCl
molarity and pH,
126
and alkaline hydrolysis of red
hake exhibited lower than 30% solubility at pH 1
to 7.
45,46
Pure fish myofibrillar protein are very in-
soluble close to their isoelectric point; therefore,
high solubility of FPH over a wide range of pH is
a very useful property for many food applica-
tions, including beverage applications. The good
solubility and good nutritive value of enzymati-
cally hydrolyzed fish protein hydrolysates also
makes them well suited to produce milk replacers
for weanling animals.
1,10,124
Presently, this is be-
ing done in Japan and France. In Japan, one com-
pany is making “bio-fish flour” by enzymatic
digestion of sardines that is used in feeds as a
milk replacer for calves and piglets.
60,126
Menha-
den hydrolysate produced by pancreatine was
found to be an excellent milk replacer with high
PER value with the process cost half as much as
dried skimmed milk.
127
Soluble FPH is an excel-
lent amino acid source for supplementing cereal
proteins
10
and to be used in bakery products, soups,
and infant formulas.
1
Pasta enriched up to 3.5% of
fish protein hydrolysate increased the protein
content by 5%, with a dramatic increase in the
dietary essential amino acids lysine (37.5%), va-
line (31%), and threonine (18%).
128
Soluble FPH
from extensive hydrolysis is an excellent source
of nitrogen for microbial growth. Extensive hy-
drolysis results in a product of free amino acids
and low-molecular-weight peptides, and there-
fore has found to be very promising for microbial
peptone production.
129
In the U.S., the main appli-
cation of FPH is not for foods but as weanling
feed for piglets, and increasingly as a pellet coat-
ing for pet food.
60
B. Water-Holding Capacity
Water-holding capacity refers to the ability
of the protein to imbibe water and retain it against
gravitational force within a protein matrix, such
as protein gels or beef and fish muscle, and it is
positively correlated with water-binding capac-
ity.
120
Water-holding capacity of proteins added
to muscle tissue is of great importance to the food
industry because retaining water in a food system
often improves texture. The functional properties
of proteins in a food system depend in part on the
water-protein interaction, and the final outcome
greatly depends on how well the protein binds
and holds water in a food system. However, a
successful specific application of a functional
protein ingredient or additive for enhancing a
single specific functional property may not be
transferable to other food systems. Fish protein
hydrolysates are highly hygroscopic, and this has
to be considered when producing them. Proper
packaging and low relative humidity of air during
processing is an important consideration. The
presence of polar groups such as COOH and NH
2
that increase during enzymatic hydrolysis have a
substantial effect on the amount of adsorbed wa-
ter and moisture sorption isotherm for these ma-
terials. The recommended maximum water con-
tent of FPH for storage is 0.075 g/g at less than
15% RH.
130
Fish protein hydrolysates have excellent wa-
ter-holding capacity, and thus useful properties
for certain food formulations. Kristinsson
90
made
salmon FPH with several enzymes to 5, 10, and
15% DH and added these to minced salmon pat-
ties at 1.5%. Water loss after freezing (48 days)
and thawing was reduced to 1% compared with
3% for the control. In this study there was no
connection observed between %DH and water
loss, FPH made using Alcalase had the best wa-
ter-holding properties in salmon mince patties,
but all FPH exhibited better water-holding prop-
erties than egg albumin and soy protein concen-
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
68
trate. Onodenalore and Shahidi
82
increased cook-
ing yield of comminuted pork by 2.4 to 9.3% by
adding 0.5 to 3.0% of a shark protein hydrolysate.
Cooking yield increased with increasing addition,
and the hydrolysates obtained via an Alcalase-
assisted process from the washed myofibrillar
shark proteins were more effective than hydroly-
sates made from untreated shark muscles. Shahidi
et al.
59
found similar results with capelin protein
hydrolysates (CPH) when added at a 3% level to
communited pork, CPH increased cooking yield
of approximately 4%. At even lower addition lev-
els there was a large reduction in the amount of
drip loss, indicating that CPH has strong water-
binding capacity. In addition, CPH at 0.5 to 3.0%
level inhibited the formation of TBARS by 17.7
to 60.4%, suggesting that CPH may have antioxi-
dant properties, perhaps due to chelation effects.
Hatate et al.
131
also found that sardine myofibril
protein hydrolysates exhibited antioxidant activ-
ity. More importantly, hydrolysates appeared to
act synergistically with several commercial anti-
oxidants. These antioxidant effects are highly
dependent on the amino acid composition and
molecular size of the hydrolysate peptides.
Fish protein concentrate (FPC) produced from
sardine with an ethanol process has also been
studied in a meat model system with respect to
water holding. FPC was added to a hamburger-
type product made from beef at 20, 40, and 60%
addition, and increased percentage of FPC in the
formulations significantly improved the cooking
yields of the products.
37
C. Emulsifying Properties
The emulsifying properties of FPH are di-
rectly connected to their surface properties, or
how effectively the hydrolysate lowers the inter-
facial tension between the hydrophobic and hy-
drophilic components in food. Proteins adsorb to
the surface of freshly formed oil droplets during
homogenization and form a protective membrane
that prevents droplets from coalescing.
6
Hydroly-
sates are surface active and promote oil-in-water
emulsions because they have hydrophilic and hy-
drophobic functional groups and are water
soluble.
121
Hydrolysates orient their hydrophobic
loops in the apolar oil phase, while the polar
segments extend into the aqueous phase. Desir-
able surface active proteins and protein hydroly-
sates have three major attributes: (1) ability to
rapidly absorb to an interface, (2) ability to rap-
idly unfold and reorient at an interface, and (3) an
ability, once at the interface, to interact with the
neighboring molecules and form a strong cohe-
sive, viscoelastic film that can withstand thermal
and mechanical motions.
120,132
Emulsifying capacity and emulsifying stabil-
ity are two methods generally used to measure the
ability of protein hydrolysates to form and stabi-
lize emulsions. Emulsifying capacity is usually
defined as the volume of oil (ml) that can be
emulsified by the protein hydrolysate (g), before
phase inversion or collapse of emulsion occurs.
31
Because FPH produces emulsions of low viscos-
ity, the most accurate way to measure this is to
use an oil titration method,
133
and measure the
electrical resistance during oil titration. A sudden
increase in resistance is observed when the maxi-
mum emulsifying capacity is reached. Emulsion
stability refers to the ability of an emulsion to
resist changes in its properties over time
134
and
can be determined simply using gravitational
methods. Measurement involves blending the
hydrolysate with oil and water, centrifuging, and
measuring total volume of emulsion, its stability
expressed as the difference between total volume
of an emulsion and the aqueous volume to total
volume.
135,136
The emulsifying properties of hydrolyzed
protein are improved by carefully controlling the
extent of hydrolysis. Extensive hydrolysis results
in a drastic loss of emulsifying properties.
58
Al-
though small peptides are highly stable and dif-
fuse rapidly and adsorb at the interface, they are
less efficient in reducing the interfacial tension
because they cannot unfold and reorient at the
interface, like proteins with higher molecular
weight.
137
Solubility seems to play an important
role in emulsification because rapid migration to
and adsorption at the interface are critical.
138
However, complete solubility is not an absolute
requirement. Solubility and emulsifying proper-
ties have been found to correlate up to 25% pro-
tein solubility.
120
Casein hydrolyzed with pancre-
atin showed a linear decrease in emulsifying
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
69
activity with increase in %DH. Casein hydroly-
sate at 67% DH consisted solely of amino acids,
di-, and tripeptides with drastically reduced emul-
sifying activity.
57
Smaller and smaller peptides
are formed as the enzymatic hydrolysis progresses
and this impacts emulsifying properties.
138
Pro-
tease specificity also plays a key role in the emul-
sifying properties of protein hydrolysates because
this strongly influences the molecular size and
hydrophobicity of the resulting peptides. Kuhler
and Stine
72
found that whey protein hydrolyzed
with Prolase yield large-molecular-weight pep-
tides with excellent emulsifying stability and ac-
tivity. In contrast, Pronase, which has a broad
specificity, produced much smaller peptides and
yielded hydrolysates with very poor emulsifica-
tion properties. Hence, a careful choice of en-
zymes and low degrees of hydrolysis are recom-
mended if good emulsifying properties are desired.
There is a relationship between %DH and
emulsifying properties for fish protein hydroly-
sates. Enzymatic hydrolysis had a negative influ-
ence on the capacity to form and stabilize emul-
sions as degree of hydrolysis increased for sardine
protein hydrolysates
83
and salmon protein hydroly-
sates.
90
Different molecular weight distributions
of the hydrolysates show that a higher content of
high-molecular-weight protein fractions plays an
important role in stabilizing emulsions. Hydroly-
sates with lower degrees of hydrolysis have higher
surface hydrophobicity and sardine hydrolysates
at low %DH have better emulsifying capacity
than commercial sodium caseinate.
83
Cui
68
ob-
tained similar results for enzymatically hydro-
lyzed chum salmon muscle.
Hydrophobicity plays an important positive
role in determining emulsifying properties. Kato
and Nakai
139
reported that effective hydrophobic-
ity determined fluorometrically showed signifi-
cant correlation with interfacial tension and emul-
sifying activity of a wide variety of proteins
studied. Li-Chan et al.
140
also found that surface
hydrophobicity predicted the emulsifying proper-
ties of meat proteins. A positive correlation be-
tween surface activity and peptide length has been
found,
73
and it has since been generally accepted
that a peptide should have a minimum length of
>20 residues to possess good emulsifying and
interfacial properties.
141
Various emulsification properties have been
found for hydrolyzed seafood protein products.
Hydrolyzed processing wastes from lobster using
papain and an undefined fungal protease had a
rather poor capacity for oil emulsification.
86
Also,
relatively poor emulsifying capacity and stability
were reported for enzymatically produced capelin
protein hydrolysate
59
and shark protein hydroly-
sates;
82
however, neither of these studied defined
the %DH of the products. Enzymatically hydro-
lyzed myofibrillar proteins from rockfish fillets
showed an increase in emulsifying capacity and
stability with hydrolysis compared to the intact
protein.
34,123
Emulsifying capacity reached a point
at which there was no change with %DH.
34
Hy-
drolysates from rockfish muscle made with bro-
melain had relatively poor emulsifying capacity
and yielded unstable emulsions.
136
Removing lip-
ids from rockfish protein hydrolysates resulted in
a dramatic loss in emulsifying capacity; the loss
increased with increasing extraction temperature.
34
Fish protein derivatives with good emulsify-
ing properties can also be prepared by acetyla-
tion.
60
Acylating agents such as acetic or succinic
anhydrides can react with the amino groups of
proteins in an alkaline environment, forming amide
groups.
24,142
The acetyl residues participate in the
formation of additional hydrogen bonds or else
can contribute to hydrophobic adherences, thus
increasing emulsifying properties. Groninger and
Miller
136,142
are among researchers that reported
an increase in emulsifying properties of fish pro-
tein by acetylation. Miller and Groninger
136
found
that emulsifying properties of FPH prepared by
bromelain increased until 43 to 59% of the free
amino groups were acetylated, with further acety-
lation having no effect.
Emulsifying properties of fish protein con-
centrates have also been reported.
27
Fish protein
concentrate produced by isopropanol extraction
had a decreased emulsifying capacity with in-
creased solvent extraction. This loss of emulsifi-
cation capacity was believed to be tied to a loss in
solubility.
The emulsifying ability of other food pro-
teins, primarily milk protein, has been studied
extensively. Enzymatically hydrolyzed whey pro-
tein had excellent emulsifying capacity and sta-
bility when made with the protease Prolase (~3.0 g
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
70
oil/mg protein).
72
Pepsin and Pronase hydrolyzed
whey protein yielded emulsions of less capacity
and stability, both decreasing with time of hy-
drolysis and %DH.
72
The degree of hydrolysis
was, however, not specified. Each of these en-
zymes have different substrate specificities and
action patterns. Pepsin and Pronase hydrolysates
had higher %DH values than Prolase hydroly-
sates. Enzymatic hydrolysis progressively de-
creased the emulsifying activity index of casein,
with a more than twofold reduction after hydroly-
sis to a %DH of 58%, with an additional small
decrease at 67% DH.
57
A drop in emulsifying
capacity was highly correlated with a drop in
hydrophobicity, and reduced molecular size, and
thus in agreement with our findings.
90
Trypsin
hydrolysis was found to greatly improve the emul-
sifying properties of casein and whey protein and
was highly influenced by pH.
138
Chicken head hydrolysates
70,71
and turkey
waste hydrolysates
44
have also been tested for
emulsifying capacity, and both had poor emulsi-
fication ability. A significant drop in emulsifying
properties as hydrolysis progressed was observed
when alpha-chymotrypsin was used to hydrolyze
peanut protein fractions, reaching a stationary
phase after a certain time.
78
There are many different factors that may
account for the difference observed between hy-
drolysates in both the ability to form an emulsion
and emulsion stability. Peptides molecular char-
acteristics and peptide chain length are the major
reason for the different emulsification ability of
hydrolysates. Environmental conditions such as
pH, ionic strength, temperature, etc. also have an
effect on the emulsification properties. Also, some
synergistic effects of peptides on emulsifying
properties have been noted.
117
In short, the fore-
going indicates that peptide behavior is complex
and not easy to explain.
D. Foaming Properties
The chemistry underlying foaming properties
of protein and protein hydrolysates have many
things in common with emulsifying properties.
Both rely on the surface properties of protein.
Food foams consist of air droplets dispersed in
and enveloped by a liquid containing a soluble
surfactant lowering the surface and interfacial
tension of the liquid.
31
The amphiphilic nature of
proteins makes this possible; the hydrophobic
portion of the protein extends into the air and the
hydrophilic portion into the aqueous phase.
Townsend and Nakai
143
showed that total hydro-
phobicity, or the hydrophobicity of exposed or
unfolded protein, have a significant correlation to
foaming formation. The surface hydrophobicity,
which is important for emulsification, does not
correlate with foam formation.
Many different methods have been developed
to measure foaming properties of proteins and
protein hydrolysates. Most, if not all, of these
methods have the drawback of poor experimental
reproducibility, thus making it hard to compare
findings between laboratories. Foaming proper-
ties are usually expressed as foam formation and
foam stability. Phillips et al.
144
developed a method
where the ability of protein to form foams is
described as overrun. Overrun is the percentage
of excess volume produced by whipping a protein
containing liquid compared with the initial vol-
ume of the liquid. Foam stability is measured by
whipping the protein solution and measuring the
time required to decrease half of the volume.
Foam stability is a highly empirical method, but
is simple to perform and has found useful appli-
cations with FPH.
Very few studies have been performed on
foaming properties of fish protein hydrolysates.
Shahidi et al.
59
and Onodenalore and Shahidi
82
studied the foam formation, or whippability, and
foam stability of shark and capelin protein hy-
drolysates, respectively. In both processes Alcalase
was the protease used for hydrolysis. The capelin
protein hydrolysate had good whippability, 90%,
at a 12% DH. Shark protein hydrolysates from
shark meat had higher whippability, 106%, and
hydrolysates from washed shark myofibrillar pro-
teins had whippability of 130%. Capelin hydroly-
sates had higher foam stability after 30 s that
dropped significantly compared with hydrolyzed
shark myofibrillar protein. Hydrolyzed whey pro-
tein isolates prepared using Alcalase had excel-
lent foaming formation and stability at a 3% DH.
145
The foaming ability for whey was much higher
than for shark and capelin protein hydrolysates.
There is a connection between the degree of
hydrolysis and foaming properties. Kuehler and
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
71
Stine
72
concluded that whey proteins hydrolyzed
to a limited degree had a increased foaming abil-
ity but reduced foam stability. This was attributed
to more air being incorporated into solution of
smaller peptides, but the smaller polypeptides do
not have the strength required to give a stable
foam. This was seen in the initial 30 min of
hydrolysis with further hydrolysis resulting in
peptides that lack the ability to stabilize the air
cells in the foam. Weak foams are commonly
observed when food proteins are hydrolyzed.
However, the advantage of using hydrolyzed pro-
teins as foaming agents is their insensitivity to
change in pH. The pH of the dispersing medium
markedly affects foaming, particularly foam sta-
bility, with foaming properties being highest close
to the isoelectric point of the protein.
146
Fish pro-
tein hydrolysates and especially fish protein con-
centrates (FPC) have the unusual property of hav-
ing good foaming properties, and of making strong,
stable foams over a wide pH range.
29-31
The foam
formation of FPC is provided by the soluble pro-
teins, only 1% of total FPC, while the remaining
denatured proteins act as foam stabilizers.
30
Foaming properties of fish protein hydroly-
sates can be improved by acylation. Groninger
and Miller
147
succinylated hydrolysates produced
by hydrolyzing rockfish fillets with bromelain.
These foams were significantly more stable com-
pared with foams from modified soy protein and
egg white; however, they had smaller foam vol-
ume. The succinylated protein foams were more
stable over a wide range of pH (3 to 9) compared
with the other protein. The succinylated FPH was
incorporated into a dessert topping, a soufflé, and
both a chilled and a frozen dessert, and the prod-
ucts were as acceptable to a taste panel as similar
foods containing no fish protein. Ostrander et
al.
148
incorporated succinylated FPH, as prepared
by Groninger and Miller,
147
into whipped gelatin
desserts. The succinylated FPH formed a stable
foam with smooth texture, highly acceptable with
respect to flavor and mouthfeel.
E. Fat Absoption
Various methods for the measurement of fat
absorption have been described. Commonly, hy-
drolysates are mixed with a specified amount of
excess fat for a particular time and then centri-
fuged at a low centrifugal force and the fat ad-
sorption expressed as the millilter of fat bound by
1 g protein hydrolysate
59,90
for FPH. The mecha-
nism of fat absorption, as assessed by this method,
is attributed mostly to physical entrapment of the
oil, and thus the higher bulk density of the protein
the more fat absorption.
31
Fat-binding capacity of
proteins correlates with surface hydrophobicity.
Fat absorption of salmon protein hydroly-
sates produced using different enzymes to 5, 10,
and 15% DH was studied by Kristinsson.
90
The
FPH at all %DH exhibited excellent fat absorp-
tion, greater than both egg albumin and soy pro-
tein concentrate. The hydrolysates at 5% DH had
significantly higher fat absorption (5.98 to 7.07 ml
oil/g FPH) than 10% DH (3.22 to5.12 ml oil/g
FPH) and 15% DH (2.86 to 3.86 ml oil/g FPH)
due to the larger peptide sizes. Only two other
publications dealing with fat absorption for FPH
could be found, for capelin protein hydrolysates
59
and shark protein hydrolysates.
82
Both studies fail
to define the units used to express fat absorption,
and thus it is impossible to compare them to other
studies.
90
Other protein hydrolysates have been studied
with respect to fat absorption. Casein hydroly-
sates with excellent oil-holding capacity, made
with papain, were dissociated by SDS, indicating
that hydrophobic interactions were primarily re-
sponsible. The substrate specificity of enzymes
also seemed to play a major role in this.
114
This
was further confirmed by Kristinsson,
90
who
showed that different enzymes used to hydrolyze
salmon muscle protein had different fat absorp-
tion ability. The capacity of a hydrolysate to ab-
sorb fat/oil is an important attribute that not only
influences the taste of the product, but it is also an
important functional characteristic that is required
especially for the meat and confectionery indus-
try. Fish protein hydrolysates therefore could very
well be used in such applications.
F. Sensory Properties
Although enzymatic hydrolysis of proteins
develops desirable functional properties, it has
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
72
the disadvantage of generating bitterness. This is
a common problem with fish protein hydroly-
sates, and the major reason for its slow accep-
tance as a food ingredient. The mechanism of
bitterness is not very clear, but it is widely ac-
cepted that hydrophobic amino acids of peptides
are a major factor. In fact, the hydrophobicity of
peptides can be measured, and it has been found
that most bitter peptides have values over 1400
kcal/mol, whereas nonbitter peptides have values
below 1300 kcal/mol. This principle is, however,
only valid for molecular weights below 6000 D.
149
Peptides with a molecular weight from 1000 to
6000 D and with hydrophobic characteristics have
shown most likely to be bitter.
102
Hydrolysis of
protein results in exposing buried hydrophobic
peptides, which will be readily able to interact
with the taste buds resulting in detection of bitter
taste. An extensive hydrolysis to free amino ac-
ids, however, decreases the bitterness of these
bitter peptides because hydrophobic peptides are
far more bitter compared with a mixture of free
amino acids. Free amino acids, are however, un-
desirable from a functional standpoint. Strict con-
trol of any hydrolysis experiment and termination
at low %DH values therefore is desirable to pre-
vent the development of a bitter taste and the
retention of functional properties.
Common features found in the structure of
bitter peptides are, for example, N, or C, terminal
basic amino acid residues, C, or N, terminal hy-
drophobic peptide fragments and peptide chain
folding due to proline residues within the peptide
structure.
150
Bitterness is due to particular arrange-
ments of certain chemical groups in the peptides.
Two functional groups are necessary to produce
bitterness, such as a pair of hydrophobic groups
or a hydrophobic or a basic group.
151
The sensa-
tion of bitterness may also be exerted by chemical
compounds having a hydrophobic region and one
hydrophilic group spaced 0.3 nm apart.
95
Because
enzymes have different preferences for amino
acids, choosing the most appropriate enzyme
preparation for hydrolysis can control the bitter-
ness. Enzymes with a high preference for hydro-
phobic amino acids such as Alcalase are often
preferred and frequently yield products of low
bitterness.
20,96
The use of exopeptidases, as op-
posed to endoproteinases, can also be helpful in
overcoming the bitterness in fish protein hydroly-
sates, particularly exopeptidases that split off
hydrophobic amino acids from bitter peptides.
However, for an enzyme preparation to be effec-
tive for hydrolyzing protein, both exopeptidases
and endoproteinases are required. Many studies
have shown that proteolytic preparations contain-
ing exopeptidases and endoproteinases produce
less bitter peptides than single proteases.
152-155
Trials have shown that by the additional action of
exopeptidases in proteolytic processes, it is pos-
sible to move the bitterness point up to higher
degree of hydrolysis.
156
Despite the problem with bitterness of fish
protein hydrolysates, little research has been con-
ducted. While FPH is highly nutritious and safe
and functional properties are good, the sensory
properties are extremely important for the suc-
cessful adaptation and acceptance by the food
industry and the consumer. Yu and Fazidah
125
found a connection between the degree of hy-
drolysis and the intensity of bitterness when hy-
drolyzing Aristichthys nobilis with a commer-
cial protease. As the %DH increased so did the
bitterness, and after a 5-h hydrolysis samples
were described as “very bitter”. Interestingly, no
bitterness was observed until after 4 h of hy-
drolysis. Papain-hydrolyzed herring had higher
bitterness scores than Alcalase-hydrolyzed her-
ring.
5
Alcalase hydrolyzed samples at higher
degree of hydrolysis were less bitter than pa-
pain-hydrolyzed samples at the same %DH. The
greater hydrophobic specificity of Alcalase com-
pared with papain could result in a reduced bitter-
ness. Enzymatically hydrolyzed ethanol-extracted
herring, with either Alcalase or papain, had very
low bitterness, suggesting that reduced bitter-
ness could be due in part to lower lipid content.
Lalasidis et al.
157
hydrolyzed cod filleting offal
with Alcalase, which produced a product with a
bitter taste. This bitter taste was eliminated when
the hydrolysate was treated further with
pancreatine, which is rich in exopeptidase activ-
ity.
Fish protein hydrolysates have been in-
corporated into food systems to evaluate their
acceptability, and sometimes with satisfactory
results.
147,148
Proteins from Oreochromis
mossambicus, a freshwater fish, were hydrolyzed
with Alcalase to produce a soluble hydrolysate
that was incorporated into crackers.
158
Sensory
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
73
evaluation showed the fried crackers with up to
10% addition of FPH were highly acceptable.
Vieira et al.
86
hydrolyzed lobster processing
waste for 5 h with several commercial enzymes
and found no difference in taste between the hy-
drolysates and a control sample, and detected no
bitterness. This is perhaps due to the extensive
hydrolysis. The hydrolysate possessed a distinct,
pleasant smell, and it was proposed for use in
various formulated food products such as flavor
enhancers. Is has long been known that proteases
can be used for recovery of flavor.
159
Baek and
Cadwallader
85
attempted to fully utilize crayfish
processing byproducts as flavorings by hydrolyz-
ing them with Optimase at optimal conditions.
They found that it is possible that the hydroly-
sates could serve as feedstocks for the production
of value-added seafood flavor extracts. Fujimaki
et al.
160
reported that a fish protein concentrate
treated with Pronase had a flavor potentiating
activity like that of monosodium glutamate (MSG)
but was accompanied by an unfavorable bitter
flavor. A low molecular acidic peptide fraction
contributed significantly to this MSG-like flavor.
The acidic peptides isolated later from the same
fish protein hydrolysate showed that at least four
dipeptides (Glu-Asp, Glu-Glu, Glu-Ser and Thr-
Ser) and five tripeptides (Asp-Glu-Ser, Glu-Asp-
Glu, Glu-Gln-Glu, Glu-Gly-Ser, and Ser-Glu-Glu)
had a flavor quantitatively resembling that of
MSG, but with weaker intensities.
161
Hevia and
Olcott
162
analyzed basic tripeptides that were re-
sponsible for bitter taste in fish protein hydroly-
sates. The tripeptides contained asparagine and
lysine as the second and C-terminal residues, re-
spectively, with the N-terminal residue leucine or
glycine.
Many techniques have been suggested to re-
duce or mask bitterness in hydrolysates, but few
of them applied to FPH. These include treating
hydrolysates with activated carbon that partly
removes bitter peptides with absorption,
59,163
ex-
tracting bitter peptides with solvents,
163
applica-
tion of further enzyme treatment with exopepti-
dases,
152
and by using the so-called plastein
reaction.
164
Chakrabarti
165
reported success in
debittering fish protein hydrolysate using ethyl
alcohol. Higher concentrations of alcohol low-
ered the bitter fraction in the hydrolysate.
The plastein reaction is among few that have
been studied with respect to fish protein hydroly-
sates. Figure 9
166
outlines the steps involved in the
plastein reaction. Heck
164
investigated the plas-
tein reaction in FPH from peptic hydrolysis and
found that it reduced the bitterness but with an
accompanied loss of FPH solubility. Some suc-
cess using the plastein reaction has been reported
with fish waste
167
and sardines.
168
Plastein can be defined as a protein substrate
that may be prepared by reversing hydrolysis with
a protease such as pepsin or papain in a concen-
trated protein hydrolysate.
60,169
The plasteins are a
mixture of peptides of varied composition and
molecular weight between 1000 and 500.000
D.
24,166
Three factors are necessary for completion
of the plastein reaction: (1) a low-molecular-weight
protein hydrolysate as a substrate, (2) a high sub-
strate concentration, and (3) a reaction environ-
ment of pH 4 to 6 regardless of the enzyme in-
volved.
164
During plastein synthesis, a high
concentration of hydrolysate (30 to 50%) is incu-
bated with an enzyme resulting in a condensation
of the peptides. As new polypeptides are formed,
they aggregate via hydrophobic associations. The
final product has a low lipid content and special
hydrophobic functional properties.
31,41,60
Tanimoto
et al.
170
have studied the mechanism of plastein
synthesis with α-chymotrypsin and found that it
proceeds in two stages. The first stage is charac-
terized by a reaction of the peptide with the hy-
droxyl group of Ser 195 that produces a peptidyl-
α-chymotrypsin complex that then undergoes
aminolysis as a result of a nucleophilic attack of
a second peptide. The plastein reaction has also
been found useful for protein recovery from ex-
tensively autolytically produced fish silage.
171
It
is strongly recommended that this process be stud-
ied further for fish protein hydrolysis.
V. FUTURE DEVELOPMENTS AND
POTENTIAL APPLICATIONS
The use of FPH as a functional food ingredi-
ent still has a long way to go until it becomes
economically feasible and accepted by industry
and consumers. However, there is a wonderful
opportunity for this to happen, due to regulations
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016

Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
74
FIGURE 9. The plastein reaction. (Adapted from Ref. 166.)
of processing waste and the abundance of small
pelagic underutilized species that will be the fron-
tiers of fisheries in this century. These factors
should stimulate the conversion of low value fish
materials into more valuable and palatable prod-
ucts. Other applications outside the food industry
may also be a feasible option for FPH. Soluble
FPH from extensive hydrolysis is an excellent
source of nitrogen for maintaining the growth of
different microorganisms. Previously, it was noted
that extensive hydrolysis of fish protein results in
a product containing free amino acids and low-
molecular-weight peptides, which is very promis-
ing for microbial peptone production.
129
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
75
The beneficial chemical composition of FPH
and FPC has also led to using these materials as
fertilizer with good results. The FPH might be too
expensive for this purpose, as added enzymes are
used, and the product is usually dried. However,
Ferreira and Hultin
104
developed an enzymatic
process using the acid protease Newlase A to
extensively hydrolyze cod frames. This process is
currently being used. The use of FPC as fertilizer
is more common, either from chemical hydrolysis
or autolysis. A successful process using second-
ary raw material from processing plants (frames
and viscera) and using the acid endogenous en-
zymes to extensively hydrolyze the material is
being operated in Washington State. The final
product is sold in bulk to local farmers, mainly
cranberry growers, and with superior results com-
pared with other commercially available fertiliz-
ers.
172
The mechanism behind the effect of the
FPH and FPC to stimulate growth and develop-
ment better than synthetic fertilizers need to be
studied further. Novel applications taking advan-
tage of plant growth-stimulating effect of FPH
and FPC could possibly be developed. An exten-
sive research program supervised by Dr. Kalidas
Shetty at the University of Massachusetts at
Amherst examines the effect of FPH on plants. In
one study the effects of using FPH to stimulate
somatic embryogenesis in Anise (Pimpinella
anisum) when compared with proline, a known
stimulator, was examined.
173
The conclusion was
that FPH could well become a proline and amino
acid substitute in plant tissue culture applications.
The positive effects of FPH due to proline and its
precursor glutamate on plant growth was con-
firmed in a recent study by the same group, where
melon (Cucumis melo L.) shoot organogenesis
was stimulated.
174
Proline and glutamate can be
obtained from FPH and potentially can be used
for value-added applications in the plant propaga-
tion industry,
174
a new arena of application for
FPH and possibly a very valuable one.
Using FPH and FPC for animal feed applica-
tions due to its good amino acid balance and high
protein content could be quite feasible. As men-
tioned previously, fish silage is used primarily for
this purpose and is essentially limited to young
animals, due to the extensive hydrolysis of the
proteins.
67
Another little-studied property of FPH is its
antioxidant potential. Studies have found power-
ful antioxidant activity in intact fish protein
131
and
in hydrolysate.
59
These effects are probably highly
dependent on the amino acid composition and
molecular size of the FPH peptides and deserve
further study.
VI. CONCLUSION
There have been several attempts to make
functional fish protein hydrolysates with enzymes,
some successful and some not. Most of these
studies are rather crude, perhaps due to the ap-
plied nature of the field, and have many short-
comings, specifically failing to compare enzymes
at the same activity level, controlling the %DH
properly and characterizing the chemical and func-
tional properties of the final products. There is
potential for these products to be produced and
sold as functional food ingredients, but at present
other applications such as plant nutrients, fertiliz-
ers, and animal feeds might be more feasible.
Standardized procedures to examine the functional
properties are needed, as well as more studies on
using endogenous enzymes to make functional
FPH. With more basic research on the molecular
level the future of FPH could be bright, especially
in light of the environmental problems facing
fisheries. It is time for FPH research to take a
new, fresher, and more scientific direction, be-
cause the field has been more or less stagnant for
the last 20 years or so.
REFERENCES
1. Rebeca, B. D., Pena-Vera, M. T., and Diaz-
Castaneda, M., Production of fish protein hydroly-
sates with bacterial proteases; Yield and nutritional
value, J. Food. Sci., 56, 309, 1991.
2. FAO, Food and Agricultural Organization Yearbook,
Fishery Statistics, Rome, Italy, 1986.
3. Mackie, I. M., Fish protein hydrolysates, Proc.
Biochem., 17(1), 26, 1982.
4. Mullally, M. M., O’Callaghan, D. M., FitzGerald,
R. J., Donnelly, W. J., and Dalton, J. P., Proteolytic
and peptidolytic activities in commercial pancreatin
protease preparations and their relationship to some
whey protein hydrolysate characteristics, J. Agric. Food
Chem., 42, 2973, 1994.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
76
5. Hoyle, N. and Merritt, J.H., Quality of fish protein
hydrolysates from herring (Clupea harengus), J. Food
Sci., 59, 76, 1994.
6. Demetriades, K., Coupland, J. N., and McClements,
D. J., Physical properties of whey protein stabilized
emulsions as related to pH and NaCl, J. Food Sci.,
62(2), 342, 1997.
7. Shiau, S-Y., Seafood Protein in human and animal
nutrition, in Seafood Proteins, ed., Sikorski, Z. E.,
Pan, B. S., and Shahidi, F., Eds., Chapman & Hall,
New York, 1994.
8. Friedman, K., Nutritional value of proteins from dif-
ferent food sources: a review, J. Agric. Food Chem.,
44(1), 6, 1996.
9. Venugopal, V., Chawla, S. P., and Nair, P. M.,
Spray dried protein powder from threadfin beam:
preparation, properties and comparison with FPC type-
B, J. Muscle Foods, 7, 55, 1996.
10. Yanez, E., Ballester, D., and Monckeberg, F., En-
zymatic fish protein hydrolyzate: chemical composi-
tion, nutritive value and use as a supplement to cereal
protein, J. Food Sci., 41, 1289, 1976.
11. Spinelli, J. and Dassow, J. A., Fish proteins: their
modification and potential uses in the food industry,
in Chemistry and Biochemistry of Marine Food Prod-
ucts, AVI Publishing Company, Westport, CT, 1982.
12. Hultin, H. O., Characteristics of Muscle Tissue, in
Food Chemistry, 2nd ed., Fennema, O. R., Ed., Marcel
Dekker Inc., New York, 1985.
13. Venugopal, V. and Shahidi, F., Thermostable water
dispersions of myofibrillar proteins from Atlantic
mackerel (Scomber scombrus), J. Food Sci., 59(2),
256, 1994.
14. Sikorski, Z. E., The myofibrillar proteins in seafood,
in Seafood Proteins, Sikorski, Z. E., Pan, B. S., and
Shahidi, F., Eds., Chapman & Hall, New York, 1994.
15. Suzuki, T., Fish and Krill Protein: Processing Tech-
nology, Applied Science, London, UK, 1981.
16. Stefansson, G. and Hultin, H. O., On the solubility
of cod muscle protein in water, J. Agric. Food. Chem.,
42, 2656, 1994.
17. Feng, Y. and Hultin, H. O., Solubility of the proteins
of mackerel light muscle at low ionic strength, J.
Food Biochem., 21, 479, 1997.
18. Zubay, G., Biochemistry, William C. Brown Publish-
ers, Dubuque, IA, 1993.
19. DeMan, J. M., Principles of Food Chemistry, Van
Nostrand Reinhold, New York, 1990.
20. Adler-Nissen, J., Enzymic Hydrolysis of Food Pro-
teins, Elsevier Applied Science Publishers, Barking,
UK, 1986.
21. Skanderby, M., Protein hydrolysates: their function-
ality and applications, Food Technol. Int. Eur., 10,
141, 1994.
22. Pigott, G. M., Enzyme modifications of fishery by-
products, in Chemistry and Biochemistry of Marine
Food Products, AVI Publishing Company, Westport,
CT, 1982.
23. Snyder, D. G., Bureau of commercial fisheries pro-
gram, Food Technol., 21(9), 70, 1967.
24. Sikorski, Z. E. and Naczk, M., Modification of tech-
nological properties of fish protein concentrates, Crit.
Rev. Food Sci. Nutr., 4, 201, 1981.
25. Cheftel, C., Ahern, M., Wang, D. I. C., and
Tannenbaum, S. R., Enzymatic solubilization of fish
protein concentrate: Batch studies applicable to con-
tinuous enzyme recycling processes, J. Agric. Food
Chem., 19, 155, 1971.
26. Mackie, I. M., Proteolytic enzymes in recovery of
proteins from fish waste, Process Biochem., 12, 12,
1974.
27. Dubrow, D. L., Kramer, A., and McPhee, A. D.,
Effects of temperature on lipid extraction and func-
tional properties of fish protein concentrate (FPC) J.
Food Sci., 38, 1012, 1973.
28. Finch, R., Whatever happened to fish protein concen-
trate?, Food Technol., 31(5), 44, 1977.
29. Sheustone, F. S., Foaming of fish protein concen-
trate, Food Preserv. Q., 13, 45, 1953.
30. Hermansson, A. M., Sivik, B., and Skjoldebrand,
C., Factors affecting solubility, foaming and swelling
of fish protein concentrate, Lebensm. Wiss. Technol.,
4, 201, 1971.
31. Kinsella, J. E., Functional properties of proteins in
foods: a survey, Crit. Rev. Food Sci. Nutr., 8(4), 219,
1976.
32. Moorjani, M. N., Nair, R. B., and Lahiry, N. L.,
Quality of fish protein concentrate prepared by direct
extraction of fish with various solvents, Food Technol.,
22(12), 1557, 1968.
33. Hale, M. B., Making fish protein concentrate by en-
zymatic hydrolysis, NOAA Technical Report NMFS
SSRF-675, US Department of Commerce, Seattle, WA,
pp. 1–31, 1972.
34. Spinelli, J., Koury, B., and Miller, R., Approaches
to the utilization of fish for the preparation of protein
isolates; enzymic modifications of myofibrillar fish
proteins, J. Food Sci., 37, 604, 1972b.
35. Quaglia, G. B. and Orban, E., Enzymic solubilisation
of proteins of sardine (Sardina pilchardus) by com-
mercial proteases, J. Sci. Food Agric., 38, 263, 1987a.
36. Quaglia, G. B. and Orban, E., Influence of the de-
gree of hydrolysis on the solubility of the protein
hydrolsyates from sardine (Sardina pilchardus), J.
Sci. Food Agric., 38, 271, 1987b.
37. Vareltzis, K., Soultos, N., Zetou, F., and Tsiaras, I.,
Proximate composition and quality of a hamburger
type product made from minced beef and fish protein
concentrate, Lebensm. Wiss. u. Technol., 23(2), 112,
1990.
38. Blenford, D. E., Protein hydrolysates: functionalities
and uses in nutritional products, Int. Food Ingr., 3, 45,
1994.
39. Webster, J. D., Ledward, D. A., and Lawrie, R. A.,
Protein hydrolysates from meat industry by-products,
Meat Sci., 7, 147, 1982.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
77
40. Loffler, A., Proteolytic enzymes: sources and appli-
cations, Food Technol., 40(1), 63, 1986.
41. Thakar, P. N., Patel, J. R., and Joshi, N. S., Protein
hydrolysates: a review, Indian J. Dairy Sci., 44(9),
557, 1991.
42. Thomas, D. and Loffler, F., Improved protein
functionalities by enzymatic treatment, Food Market.
Technol., 2, 1994.
43. Orlova, T. A., Nelichik, N. N., and Fleider, K. A.,
[Edible protein concentrate from fish raw material],
Rybnoe Khozyaistvo, 10, 59, 1979.
44. Fonkwe, L. G. and Singh, R. K., Protein recovery
from enzymatically deboned turkey residue by enzy-
mic hydrolysis, Process Biochem., 31(6), 605, 1996.
45. Tannenbaum, S. R., Ahern, M., and Bates, R. P.,
Solubilization of fish protein concentrate. I. An alka-
line process, Food Technol., 24(5), 604, 1970a.
46. Tannenbaum, S. R., Ahern, M., and Bates, R. P.,
Solubilization of fish protein concentrate. II. Utiliza-
tion of the alkaline-process product, Food Tecnol.,
24(5), 607, 1970b.
47. Linder, M., Fanni, J., Parmentier, M., Sergent, M.,
and Phan-Than-Luu, R., Protein recovery from veal
bones by enzymatic hydrolysis, J. Food Sci., 60(5),
949, 1995.
48. Lahl, W. J. and Braun, S. D., Enzymatic production
of protein hydrolysates for food use, Food Technol.,
58(10), 68, 1994.
49. Krause, W. and Schmidt, K., [Studies on the enzy-
matic hydrolysis of alkali-treated milk proteins],
Untersuchungen zur enzymatischen hydrolyse von
alkalibehandelten milchproteinen, Nahrung, 18(8),
833, 1974.
50. Ledward, D. A. and Lawrie, R. A., Recovery and
utilization of by-product proteins of the meat industry,
J. Chem. Tech. Biotechnol., 34B, 223, 1984.
51. Richardson, T. and Hyslop, D. B., Enzymes, in Food
Chemistry, 2nd ed., Fennema, O. R., Ed., Marcel
Dekker Inc., New York, 1984.
52. Godfrey, T. and Reichelt, J., Eds., Industrial Enzy-
mology. The Application of Enzymes in Industry,
MacMillan, London, UK, 1983.
53. Chreighton, T. E., Proteins: Structures and Molecu-
lar Properties, W.H. Freeman & Co., New York,
1993.
54. Svendsen, I., Chemical modifications of the subtilisin
with special reference to the binding of large sub-
strates. A review, Carlsberg Res. Commun., 41, 237,
1976.
55. Phillips, R. D. and Beuchat, L. R., Enzyme modifi-
cation of proteins, in Protein Functionality in Foods,
Cherry, J. P., Ed., American Chemical Society, Sym-
posium Series 147, Washington, DC, 1981.
56. Kester, J. J. and Richardson, T., Modification of
whey protein by trypsin, J. Dairy Sci., 67, 2757, 1984.
57. Mahmoud, M. I., Malone, W. T., and Cordle, C. T.,
Enzymatic hydrolysis of casein: effect of degree of
hydrolysis on antigenicity and physical properties, J.
Food Sci., 57(5), 1223, 1992.
58. Mahmoud, M. I., Physicochemical and functional
properties of protein hydrolysates in nutritional prod-
ucts, Food Technol., 58(10), 89, 1994.
59. Shahidi, F., Han, X-Q., and Synowiecki, J., Produc-
tion and characteristics of protein hydrolysates from
capelin (Mallotus villosus), Food Chem., 53, 285,
1995.
60. Gildberg, A., Enzymic processing of marine raw
materials, Process Biochem., 28, 1, 1993.
61. Owens, J. D. and Mendoza, L. S., Enzymatically
hydrolysed and bacterially fermented fishery prod-
ucts, J. Fd. Technol., 20, 273, 1985.
62. Gildberg, A., Espejo-Hermes, J., and Magno-
Orejana, F., Acceleration of autolysis during fish
sauce fermentation by adding acid and reducing the
salt content, J. Sci. Food Agric., 35(12), 1363, 1984.
63. Raa, J. and Gildberg, A., Autolysis and proteolytic
activity of cod viscera, J. Fd. Technol., 11, 619, 1976.
64. van Veen, A. G. and Steinkraus, K. H., Nutritive
value and wholesomeness of fermented foods, J. Agric.
Food Chem., 18(4), 576, 1970.
65. Raa, J. and Gildberg, A., Fish silage: a review, CRC
Crit. Rev. Food Sci. Nutr., 14, 383, 1982.
66. van Wyk, H. J. and Heydenrych, C. M. S., The
production of naturally fermented fish silage using
various lactobacilli and different carbohydrate sources,
J. Sci. Food Agric., 36, 1093, 1985.
67. Dong, F. M., Fairgrieve, W. T., Skonberg, D. I.,
and Rasco, B. A., Preparation and nutrient analyses
of lactic acid bacteria ensiled salmon viscera, Aquac-
ulture, 109, 351, 1993.
68. Cui, H. The Influence of Degree of Hydrolysis on the
Structural and Functional Properties of Fish Protein
Peptic Hydrolysates, Masters thesis, University of
Washington, Seattle, WA, 1996.
69. Gonzalez-Tello, P., Camacho, F., Jurado, E., Paez,
M. P., and Guadix, E. M., Enzymatic hydrolysis of
whey proteins. I. Kinetic models, Biotechnol. Bioeng.,
44, 523, 1994a.
70. Surowka, K. and Fik, M., Studies on the recovery of
proteinaceous substances from chicken heads. I. An
application of neutrase to the production of protein
hydrolysate, Int. J. Food Sci. Technol., 27, 9, 1992.
71. Surowka, K. and Fik, M., Studies on the recovery of
proteinaceous substances from chicken heads. II.
Application of pepsin to the production of protein
hydrolysates, J. Sci. Food Agric., 65, 289, 1994.
72. Kuehler, C. A. and Stine, C. M., Effect of enzymatic
hydrolysis on some functional properties of whey
protein, J. Food Sci., 39, 379, 1974.
73. Jost, R. and Monti, J. C., Partial enzymatic hydroly-
sis of whey protein by trypsin, J. Dairy Sci., 60, 1387,
1977.
74. Vegarud, G. E., Svenning, C., Molland, T., and
Langsrud, T., Enzymatic hydrolysis of milk proteins
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
78
improved functional prperties, Med. Fac. Landbouww.
Rijksuniv. Gent, 56(4a), 1649, 1991.
75. Mutilangi, W. A. M., Panyam, D., and Kilara, A.,
Hydrolysates from proteolysis of heat-denatured whey
proteins, J. Food Sci., 60(5), 1104, 1995.
76. Lin, S-B., Chiang, W-E., Cordle, C. T., and
Thomas, R. L., Functional and immunological prop-
erties of casein hydrolysate produced from a two-
stage membrane system., J. Food Sci., 62(3), 480,
1997.
77. Parrado, J., Millan, F., Hernandez-Pinzon, I.,
Bautista, J., and Machado, A., Characterization of
enzymatic sunflower protein hydrolysates, J. Agric.
Food Chem., 41(11), 1821, 1993.
78. Monteiro, P. V. and Prakash, V., Alteration of func-
tional properties of peanut (Arachis hypogaea L.) pro-
tein fractions by chemical and enzymatic modifica-
tions, J. Food Sci. Technol., 33(1), 19, 1996.
79. Sen, D. P., Sripathy, N. V., Lahiry, N. L.,
Sreenivasan, A., and Subrahmanyan, V., Fish hy-
drolysates. I. Rate of hydrolysis of fish flesh with
papain, Food Technol., 5, 1962.
80. Sen, D. P., Sripathy, N. V., Lahiry, N. L.,
Sreenivasan, A., and Subrahmanyan, V., Fish hy-
drolysates. II. Standardization of digestion conditions
for preparation of hydrolysates rich in peptones and
proteoses, Food Technol., 5, 1962.
81. Limonta, B. A., Behnke, U., and Ruttloff, H., [Manu-
facture of fish protein hydrolysate] Herstellung von
fischproteinhydrolysate, German Democratic Repub-
lic Patent no. 148437, 1981.
82. Onodenalore, A. C. and Shahidi, F., Protein disper-
sions and hydrolysates from shark (Isurus oxyrinchus),
J. Aquat. Food Prod. Technol., 5, 43, 1996.
83. Quaglia, G. B. and Orban, E., Influence of enzy-
matic hydrolysis on structure and emulsifying proper-
ties of sardine (Sardina pilchardus) protein hydroly-
sates, J. Food Sci., 55(6), 1571, 1990.
84. Sugiyama, K., Egawa, M., Onzuka, H., and Oba,
K., Characteristics of sardine muscle hydrolysates
prepared by various enzymic treatments, Nippon Suisan
Gakkaishi, 57(3), 475, 1991.
85. Baek, H. H. and Cadwallader, K. R., Enzymatic
hydrolysis of crayfish processing by-products, J. Food
Sci., 60, 929, 1995.
86. Viera, G. H. F., Martin, A. M., Saker-Sampaiao,
S., Omar, S., and Goncalves, R. C. F., Studies on the
enzymatic hydrolysis of Brazilian lobster (Panulirus
spp.) processing wastes, J. Sci. Food Agric., 69, 61,
1995.
87. Sakai, N., Noyori, M., Matsunaga, H., and
Hanzawa, T., A kinetic study on the hydrolysis of
fish protein by acid protease, Nippon Shokuhin Kogaku
Kaishi, 42(5), 301, 1995.
88. Diniz, F. M. and Martin, A. M., Use of response
surface methodology to describe the combined effects
of pH, temperature and E/S ratio on the hydrolysis of
dogfish (Squalus acanthias) muscle, Int. J. Food Sci.
Technol., 31, 419, 1996.
89. Benjakul, B. and Morrissey, M. T., Protein hydroly-
sates from Pacific whiting solid wastes, J. Agric. Food
Chem., 45, 3424, 1997.
90. Kristinsson, H. G., Reaction Kinetics, Biochemical
and Functional Properties of Salmon Muscle Proteins
Hydrolyzed by Different Alkaline Proteases, Masters
thesis, University of Washington, Seattle, WA,
1998.
91. Deeslie, W. D. and Cheryan, M., Functional proper-
ties of soy protein hydrolsyates from a continous ul-
trafiltration reactor, J. Agric. Food Chem., 36(1), 26,
1988.
92. Unido, Sectoral Studies Branch, Industrial develop-
ment strategies for fishery systems in developing coun-
tries, Food Rev. Int., 6, 1, 1990.
93. Ritchie, A. H. and Mackie, I. M., Preparation of fish
protein hydrolysates, Anim. Feed Sci. Technol., 7(2),
125, 1982.
94. FAO, Protein Advisory Group, Bulletin 12, 1971.
95. Adler-Nissen, J., Control of the proteolytic reaction
and the level of bitterness in protein hydrolysis pro-
cesses, J. Chem. Technol. Biotechnol., 34B, 215, 1984.
96. Petersen, B. R., Removing bitterness from protein
hydrolysates, Food Technol., 58(10), 96, 1994.
97. Hale, M. B., Relative activities of commercially-avail-
able enzymes in the hydrolysis of fish proteins, Food
Technol., 23, 107, 1969.
98. Arzu, A., Mayorga, H., Gonzales, J., and Rolz, C.,
Enzymatic hydrolysis of cottonseed protein, J. Agric.
Food Chem., 20, 805, 1972.
99. Kristinsson, H. G. and Rasco, B. A., Kinetics of
enzymatic hydrolysis of Atlantic salmon (Salmo salar)
muscle proteins by alkaline proteases and a visceral
serine protease mixture, Proc. Biochem., submitted,
2000.
100. Liu, L. L. and Pigott, G. M., Preparation and use of
inexpensive crude pepsin for enzyme hydrolysis of
fish, J. Food Sci., 46, 1569, 1981.
101. Tarky, W., Agarwala, O. P., and Pigott, G. M.,
Protein hydrolysate from fish waste, J. Food Sci., 38,
917, 1973.
102. Gonzalez-Tello, P., Camacho, F., Jurado, E., Paez,
M. P., and Guadix, E. M., Enzymatic hydrolysis of
whey proteins. II. Molecular-weight range, Biotechnol.
Bioeng., 44, 529, 1994b.
103. Beddows C. G. and Ardeshir, A. G., The production
of soluble fish protein solution for use in fish sauce
manufacture, J. Fd. Technol., 14, 603, 1979
104. Ferreira, N. G. and Hultin, H. O., Liquefying cod
fish frames under acidic conditions with a fungal en-
zyme, J. Food Proc. Pres., 18, 87, 1994.
105. Mullally, M. M., O’Callaghan, D. M., FitzGerald,
R. J., Donnelly, W. J., and Dalton, J. P., Zymogen
activation in pancreatic endoproteolytic preparations
and influence on some whey protein characteristics, J.
Food Sci., 60(2), 227, 1995.
106. Hevia, P., Whiteker, J. R., and Olcott, H. S., Solu-
bilization of a fish protein concetrate with proteolytic
enzymes, J. Agric. Food Chem., 24(2), 383, 1976.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
79
107. Moreno, M., M., C. and Cuadrado, V. F., Enzymic
hydrolysis of vegetable proteins: mechanism and ki-
netics, Process Biochem., 28, 481, 1993.
108. Archer, M. C., Ragnarsson, J. O., Tannenbaum, S.
R., and Wang, D. I. C., Enzymatic solubilization of
an insoluble substrate, fish protein concentrate: pro-
cess and kinetic considerations, Biotechnol. Bioeng.,
15, 181, 1973.
109. Langmyhr, E., Enzymatic hydrolysis of fish protein,
Dissertation Abstracts International, 43(4), 758, 1981.
110. Adler-Nissen, J., Enzymatic hydrolysis of food pro-
teins, Process Biochem., 12(6), 18, 1977.
111. Steinhardt, H. and Beychok, S., Interaction of pro-
tein with hydrogen ions and other small ions and
molecules, in The Proteins, Vol. 2, Neurath, H., Ed.,
Academic Press, New York, 1964.
112. Adler-Nissen, J., Determination of the degree of
hydrolysis of food protein hydrolysates by trinitro-
benzenesulfonic acid, J. Agric. Food Chem., 27(6),
1256, 1979.
113. Ukeda, H., Matsumoto, K., Katsuki, I., and
Osajima, Y., A simple method for monitoring the
enzymatic hydrolysis of fish meat: determination of
amino groups using gluteraldehyde, Nippon
Nogeikagaku Kaishi, 65(8), 1229, 1991.
114. Haque, Z. U., Influence of milk peptides in determin-
ing the functionality of milk proteins: a review, J.
Dairy Sci., 76(1), 311, 1993.
115. Deeslie, W. D. and Cheryan, M., Fractionation of
soy protein hydrolysates using ultrafiltration mem-
branes, J. Food Sci., 57(2), 411, 1991.
116. Van Beresteijn, E. C. H, Peeters, R. A., Kaper, J.,
Meijer, R. J. G. M., Robben, A. J. P. M., and
Schmidt, D. G., Molecular mass distribution, immu-
nological properties and nutritive value of whey pro-
tein hydrolysates, J. Food Protection, 57(7), 619, 1994.
117. Gauthier, S. F., Paquin, P., Pouliot, Y., and
Turgeon, S., Surface activity and related functional
properties of peptides obtained from whey proteins, J.
Dairy Sci., 76(1), 321, 1993.
118. Haque, Z. U., Importance of peptides for food protein
functionality, in Food Polymers, Gels and Colloids,
Royalty Society of Chemistry London, England,
1991.
119. Adler-Nissen, J. and Olsen, H., The influence of
peptide chain length on taste and functional properties
of enzymatically modified soy protein, in Food Chem-
istry, Pour-El, A., Ed., American Chemical Society,
Washington, D.C., 1979.
120. Damodaran, S., Amino acids, peptides, and proteins,
in Food Chemistry, 3rd ed., Fennema, O. R., Ed.,
Marcel Dekker Inc., New York, 1996.
121. Wilding, P., Lilliford, P. J., and Regenstein, J. M.,
Functional properties of proteins in foods, J. Chem.
Technol. Biotechnol., 34B, 182, 1984.
122. Morr, V., German, B., and Kinsella, J. E.,
Regenstein, J. M., Van Buren, J. P., Kilara, A.,
Lewis, B. A., and Mangino, M. E., A collaborative
study to develop a standardized food protein solubil-
ity procedure, J. Food Sci., 50, 1715, 1985.
123. Spinelli, J., Koury, B., and Miller, R., Approaches
to the utilization of fish for the preparation of protein
isolates: Isolation and properties of myofibrillar and
sarcoplasmic fish protein, J. Food Sci., 37, 599,
1972a.
124. McNairney, J., Modification of a novel protein prod-
uct, J. Chem. Technol. Biotecnol., 34B, 206, 1984.
125. Yu, S. Y. and Fazidah, S., Enzymic hydrolysis of
proteins from Aristichthys noblis by protease P
“Amano” 3, Trop. Sci., 34, 381, 1994.
126. Venugopal, V. and Shahidi, F., Value-added prod-
ucts from underutilized fish species, Crit. Rev. Food
Sci. Nutr., 35(5), 431, 1995.
127. Hale, M. B. and Bauersfeld, Jr., P. E., Preparation
of a menhaden hydrolysate for possible use in a milk
replacer, Mar. Fish. Rev., 40(8), 14, 1978.
128. Mynov, V. A. and Kim, L. V., [Enrichment of pasta
with fish protein hydrolysates], Khlebopekarnaya I
Konditerskaya Promyshlennost, 5, 43, 1980.
129. Skorupa, K. and Sikorski, Z. E., Mozliwosci
wykorzystania mniej cennych surowcow rybnych do
otrzymywania peptonow mivrobiologicznych,
Przemysl spozywczy, 6, 159, 1993.
130. Buinov, A. A., Ginzburg, A. S., and Syroedov, V. I.,
[Hygroscopic properties of fish protein hydrolysates,
dried as a foam], Izvestiya Vysshikh Uchebnykh
Zavedenii, Pishchevaya Technologiya, 3, 110, 1977.
131. Hatate, H., Numata, Y., and Kochi, M., Synergistic
effect of sardine myofibril protein hydrolyzates with
antioxidant, Nippon Suisan Gakkaishi, 56(6), 1011,
1990.
132. Phillips, M. C., Protein conformation at liquid inter-
faces and its role in stabilizing emulsions and foams,
Food Technol., 35, 50, 1981.
133. Webb, N. B., Ivey, F. J., and Craig, H. B., The
measurement of emulsifying capacity by electric re-
sistance, J. Food Sci., 35, 501, 1970.
134. McClements, D. J., Food Emulsions: Principles,
Practice and Techniques, CRC Press, Boca Raton,
FL, 1999.
135. Yatsumatsu, K., Sawada, K., and Moritaka, S.,
Whipping and emulsifying properties of soybean prod-
ucts, Agric. Biol. Chem.(Japan), 36(5), 719, 1972.
136. Miller, R. and Groninger, H. S., Functional proper-
ties of enzyme-modified acylated fish protein deriva-
tives, J. Food Sci., 41, 268, 1976.
137. Turgeon, S. L., Gauthier, S. F., and Paquin, P.,
Interfacial and emulsifying properties of whey pep-
tide fractions obtained with a two step ultrafiltration
process, J. Agric. Food Chem., 39, 637, 1991.
138. Chobert, J-M., Bertrand-Harb, C., and Nicolas,
M-G., Solubility and emulsifying properties of caseins
and whey proteins modified enzymatically by trypsin,
J. Agric. Food Chem., 36(5), 883, 1988.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
80
139. Kato A. and Nakai, S., Hydrophobicity determined
by a fluorescence probe method and its correlation
with surface properties of proteins, Biochim. Biophys.
Acta, 13, 624, 1980.
140. Li-Chan, E., Nakai, S., and Wood, D. F., Hydropho-
bicity and solubility of meat proteins and their rela-
tionship to emulsifying properties, J. Food Sci., 49,
354, 1984.
141. Lee, S. W., Shimizu, M., Kaminogawa, S., and
Yamaguchi, K., Emulsifying properties of a mixture
of peptides derived from the enzymatic hydrolysates
of β-casein, Agric. Biol. Chem., 51, 161, 1987.
142. Groninger, H. S. and Miller, R., Some chemical and
nutritional properties of acylated fish proteins, J. Agric.
Food Chem., 27(5), 949, 1979.
143. Townsend, A. A. and Nakai, S., Relationships be-
tween hydrophobicity and foaming characteristics of
food proteins, J. Food Sci., 48, 588, 1983.
144. Phillips, L. G., Haque, Z., and Kinsella, J. E., A
method for the measurement of foam formation and
stability, J. Food Sci., 52, 1074, 1987.
145. Althouse, P. J., Dinakar, P., and Kilara, A., Screen-
ing of proteolytic enzymes to enhance foaming of
whey protein isolates, J. Food Sci., 60(5), 1110,
1995.
146. Kinsella, J. E., Functional properties of proteins:
possible relationships between structure and function
in foams, Food Chem., 7, 273, 1981.
147. Groninger, H. S. and Miller, R., Preparation and
aeration properties of an enzyme-modified succinylated
fish protein, J. Food Sci., 40, 327, 1975.
148. Ostrander, J. G., Nystrom, P. J., and Martinsen,
C. S., Utilization of a fish protein isolate in whipped
gelatine desserts, J. Food Sci., 42(2), 559, 1977.
149. Petersen, B. R., The impact of the enzymatic hy-
drolysis process on recovery and use of proteins, in
Enzymes and Food Processing, Elsevier Applied Sci-
ence Publishers, London, UK, 1981, 149–175.
150. Yeom, H. W., Kim, K. S., and Rhee, J. S., Soy
protein hydrolysate debittering by lysine-acetylation,
J. Food Sci., 59(5), 1123, 1994.
151. Tamura, M., Mori, N., Miyoshi, T., Koyama, S.,
Kohri, H., and Okai, H., Practical debittering using
model peptides and related compounds, Agric.
Biol.Chem., 54(1), 41, 1990.
152. Clegg, K. M. and McMillan, A. D., Dietary enzymic
hydrolysates of protein with reduced bitterness, J.
Food Technol., 9, 21, 1974.
153. Cogan, V., Moshe, M., and Mokady, S., Debittering
and nutritional upgrading of enzymic casein hydroly-
sates, J. Sci. Food. Agric., 32, 459, 1981.
154. Vegarud, G. E. and Langsrud, The level of bitter-
ness and solubility of hydrolysates produced by con-
trolled hydrolysis of caseins, J. Dairy Res., 56, 375,
1989.
155. Moll, D., Manufacturing protein hydrolysates without
giving use to a bitter taste, in Food Ingredients Eu-
rope Conference, Expoconsult Publishers, Maarssen,
The Netherlands, 1990, 257–260.
156. Przybyla, A. E., Enzymes used for protein hydroly-
sate debittering; Enzymatic hydrolysis of proteins to
produce flavorings, dietary supplements and other
products that can result in a bitter taste, Food Eng., 9,
51, 1989.
157. Lalasidis, G., Bostrom, S., and Sjoberg, L.-B., Low-
molecular-weight enzymatic fish protein hydrolysates:
Chemical composition and nutritive value, J. Agric.
Food Chem., 26(3), 751, 1978.
158. Yu, S. Y. and Tan, L. K., Acceptability of crackers
(‘Keropok’) with fish protein hydrolsyates, Int. J. Food
Sci. Technol., 25(2), 204, 1990.
159. Haard, N. F., A review of proteolytic enzymes from
marine organisms and their application in the food
industry, J. Aquat. Food Prod. Technol., 1(1),
1992.
160. Fujimaki, M., Arai, S., Yamashita, M., Kato, H.,
and Nogushi, M., Taste peptide fractionation from a
fish protein hydrolysate, Agric. Biol. Chem. (Japan),
37, 2891, 1973.
161. Noguchi, M., Arai, S., Yamashita, M., Kato, H.,
and Fujimaki, M., Isolation and identification of
acidic oligopeptides in a flavor potentiating fraction
from a fish protein hydrolysate, J. Agric. Food Chem.,
23(1), 49, 1975.
162. Hevia, P. and Olcott, H. S., Flavor of enzyme-solu-
bilized fish protein concentrate fractions, J. Agric.
Food Chem., 25(4), 772, 1977.
163. Lalasidis, G., Four new methods of debittering
protein hydrolysates and a fraction of hydrolysates
with high content of essential amino acids, Annales
de la Nutrition et de l’Alimentation, 32(2/3), 709,
1978.
164. Heck, N. E., Characterization of Fish Protein Hy-
drolysate Plastein and the Identification of Glutamyl-
Lysine in the Plastein Material, Ph.D. disseration,
University of Washington, Seattle, WA, 1983.
165. Chakrabarti, R., A method of debittering fish pro-
tein hydrolysate, J. Food Sci. Technol., 20(4), 154,
1983.
166. Arai, S., Yamashita, M., and Fujimaki, M., Plastein
reaction and its application, Cereal Foods World, 20(2),
1975.
167. Onoue, M. and Riddle, L. M., Use of plastein reac-
tion in recovering protein from fish waste, J. Fish.
Res. Board (Canada), 30, 1745, 1973.
168. Kim, S. K. and Lee, E. H., Enzymatic modification
of sardine protein concentrate, J. Korean Agric. Chem.
Soc., 30, 234, 1987
169. Montecalvo, J., Jr., Constantinides, S. M., and
Yang, C. S. T., Enzymatic modification of fish frame
protein isolate, J. Food Sci., 49, 1305, 1984.
170. Tanimoto, S., Yamashita, M., Arai, and Fujimaki,
M., Probes for catalytic action of α-chymotrypsin in
plastein synthesis, Agric. Biol. Chem. (Japan), 36,
1595, 1972.
171. Raghunath, M. R. and McCurdy, A. R., Synthesis
of plastein from fish silage, J. Sci. Food Agric., 54,
655, 1991.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
Copyright
©
2000, CRC Press LLC — Files may be downloaded for personal use only. Reproduction of this material without
the consent of the publisher is prohibited.
81
172. Pigott, G. M., University of Washington at Seattle,
personal communication, 1998.
173. Eguschi, Y., Bela, J. S., and Shetty, K., Simulation
of somatic embryogenesis in Anise (Pimpinella
anisium) using fish protein hydrolysates and proline,
J. Herbs and Spices, 5(3), 61, 1997.
174. Milazzo, M. C., Zheng, Z., Kellett, G., Haynesworth,
K., and Shetty, K., Stimulation of benzyladenine-
induced in vitro shoot organogenesis and endogenous
proline in melon (Cucumis melo L.) by fish protein
hydrolysates in combination with proline analogues,
J. Agric. Food Chem., 47(4), 1771, 1999.
Downloaded by [Texas A&M University Libraries] at 11:28 12 January 2016
