
REFERENCE SUFFICIENCY RANGES
FOR PLANT ANALYSIS
IN THE SOUTHERN REGION
OF THE UNITED STATES
Southern Cooperative Series Bulletin #394
July 2000
Updated and reformatted July 2009
Updated September 2011
URL: www.ncagr.gov/agronomi/saaesd/scsb394.pdf
Contact information:
North North Carolina Department of Agriculture and Consumer Services Agronomic Division
4300 Reedy Creek Road, Raleigh, NC
1040 Mail Service Center, Raleigh, NC 27699-1040
(919) 733-2655
ISBN: 1-58161-394-6

xxxxx

i
July 2000 SCSB #394
REFERENCE SUFFICIENCY RANGES
FOR PLANT ANALYSIS
IN THE SOUTHERN REGION
OF THE UNITED STATES
Editor
C. Ray Campbell
For a complete list of regional project members and contributing authors,
see the List of Contributing Authors on the following pages.
Abstract
Plant analysis is a chemical evaluation of nutritional status. Concentrations of
essential elements found in indicator tissue reect the nutritional status of plants.
Proper interpretation of plant analysis results is critical to effective use of this management
tool. Guidelines for interpretation of analytical results have been developed over years based
on research, surveys, and experience. Plant analysis continues to evolve as an important
management tool as interpretive databases for various crops, stages of growth, and indicator
tissue are developed.
Reliability of interpretive guidelines vary with extent of research conducted on various crops.
This bulletin provides an overview of available interpretive information for most economically
important crops. In some cases, sufciency ranges are based on surveys and experience, while in
other cases, there are signicant research studies that can be cited. Interpretations of important
ratios of essential elements are reported as available. DRIS interpretation norms are provided for
crops as they are reported in the literature.
The overview of sufciency ranges and other interpretive data identies voids in the research
base and additional work needed to improve plant analysis. This bulletin is designed to be a work
in progress. The information provides a starting place from which improved sufciency ranges
can be developed. Revisions will be published as additional information becomes available.

ii
List of Contributing Authors
Author & E-mail Address Afliation
Baker, W. H.
Assistant Professor
Soil Testing & Research Laboratory
University of Arkansas
Marianna, AR 72360
Bell, P. F.
Assistant Professor
Dept. of Agronomy
Louisiana State University
Baton Rouge, LA 70803-2110
Campbell, C. R.
former Section Chief, Plant/Waste/Solution Analysis
Agronomic Division
N.C. Dept. Agric. & Consumer Services
Raleigh, NC 27607-6465
Cox, F. R.
former Professor
Dept. of Soil Science
North Carolina State University
Raleigh, NC 27695-7619
Donohue, S. J.
Professor & Extension Specialist
Dept. of Crop and Soil Environmental Sciences
Virginia Tech
Blacksburg, VA 24061-0403
Gascho, G. J.
Professor
Dept. of Crop & Soil Sciences, UGA
Coastal Plain Experiment Station
Tifton, GA 31793-0748
Hanlon, E. A.
hanlon@gnv.ifas.u.edu
Professor & Center Director
Southwest Florida Research & Education Center
University of Florida
Immokalee, FL 34142
Hinesley, L. E.
Professor
Dept. of Horticultural Science
North Carolina State University
Raleigh, NC 27695-7609
Hochmuth, G. J.
gjh@gnv.ifas.u.edu
Associate Professor
Dept. of Horticultural Sciences
University of Florida
Gainesville, FL 32611-0609

iii
Author & E-mail Address Afliation
Kovar, J. L.
former Associate Professor
Dept. of Agronomy
Louisiana State University
Baton Rouge, LA 70803-2110
Lessman, G. M.
Associate Professor
Dept. of Plant & Soil Science
University of Tennessee
Knoxville, TN 37996
Lippert, R. M.
Assistant Professor
Dept. of Crop & Soil Environmental Science
Clemson University
Clemson, SC 29634-0359
Miner, G. S.
former Professor
Dept. of Soil Science
North Carolina State University
Raleigh, NC 27695-7625
Mitchell, C. C.
Extension Specialist & Professor
Dept. of Agronomy & Soils
Auburn University
Auburn, AL 36849
Plank, C. O.
Associate Professor & Extension Agronomist
Dept. of Crop & Soil Sciences
Univ. of Georgia Coop. Ext. Serv.
Athens, GA 30602-7272
Sabbe, W. E.
Professor
Dept. of Agronomy
University of Arkansas
Fayetteville, AR 72703
Savoy, H. J.
Associate Professor
Dept. of Biosystems Engineering & Environmental Science
University of Tennessee
Knoxville, TN 37996
Thom, W. O.
Professor
Dept. of Agronomy
University of Kentucky
Lexington, KY 40546
Tucker, M. R. former Section Chief, Soil Testing
Agronomic Division
N.C. Dept. Agric. & Consumer Services
Raleigh, NC 27607-6465
Unruh, L. former Assistant Professor & Extension Specialist
Dept. of Soil & Crop Science
Texas A&M University
College Station, TX 77843

iv
Participating Agricultural Experiment Stations
Alabama Agric. Exp. Sta.
Auburn University
Auburn, AL 36849-5403
L. Waters, Director
Oklahoma Agric. Exp. Sta.
Oklahoma State University
Stillwater, OK 74078-0500
C. B. Browning, Director
Arkansas Agric. Exp. Sta.
University of Arkansas
Fayetteville, AR 72701
G. J. Musick, Director
Puerto Rico Agric. Exp. Sta.
University of Puerto Rico
Mayaguez, PR 00708
J. A. Quinones, Acting Director
Florida Institute of Food and Agric. Sci.
University of Florida
Gainesville, FL 32611
J. M. Davidson, Director
South Carolina Agric. Exp. Sta.
Clemson University
Clemson, SC 29634-0351
J. R. Fischer, Director
Georgia Agric. Exp. Sta.
University of Georgia
Athens, GA 30602
C. W. Donoho, Jr., Director
Tennessee Agric. Exp. Sta.
University of Tennessee
Knoxville, TN 37901
D. O. Richardson, Director
Kentucky Agric. Exp. Sta.
University of Kentucky
Lexington, KY 40546-0091
C. O. Little, Director
Texas Agric. Exp. Sta.
Texas A&M University System
College Station, TX 77843-2147
R. G. Merrield, Director
Lousiana Agric. Exp. Sta.
Louisiana State University & A&M College
Baton Rouge, LA 70894
K. W. Tipton, Director
Virginia Agric. Exp. Sta.
Virginia Polytechnic Institute & State University
Blacksburg, VA 24061-0402
L. A. Swiger, Director
Mississippi Agric. & Forest. Exp. Sta.
Mississippi State University
Mississippi State, MS 39762
V. G. Hurt, Director
Germplasm Introduction & Research Unit
USDA-ARS-GIRU
St. Croix, USVI 00851-3008
North Carolina Agric. Exp. Sta.
North Carolina State University
Raleigh, NC 27695-7643
J. C. Wynne, Director

v
Participating State Extension Services
Alabama Coop. Ext. Serv.
Auburn University
Auburn, AL 36849
S. Jones, Director
Oklahoma Coop. Ext. Serv.
Oklahoma State University
Stillwater, OK 74078-0500
C. B. Browning, Director
Arkansas Coop. Ext. Serv.
University of Arkansas
Little Rock, AR 72203
D. F. Foster, Director
Puerto Rico Coop. Ext. Serv.
University of Puerto Rico
Mayaguez, PR 00708
J. A. Quinones, Acting Director
Florida Coop. Ext. Serv.
University of Florida
Gainesville, FL 32611
Christine Waddill, Director
South Carolina Coop. Ext. Serv.
Clemson University
Clemson, SC 29634
B. K. Webb, Director
Georgia Coop. Ext. Serv.
University of Georgia
Athens, GA 30602
R. W. Isaac, Director
Tennessee Agric. Ext. Serv.
University of Tennessee
Knoxville, TN 37901
B. G. Hicks, Director
Kentucky Coop. Ext. Serv.
University of Kentucky
Lexington, KY 40546
C. O. Little, Director
Texas Agric. Ext. Serv.
Texas A&M University System
College Station, TX 77843
Z. L. Carpenter, Director
Lousiana Coop. Ext. Serv.
Louisiana State University
Baton Rouge, LA 70803-1900
D. T. Loupe, Director
Virginia Coop. Ext. Serv.
Virginia Polytechnic Institute & State University
Blacksburg, VA 24061
J. F. Johnson, Director
Mississippi State University Ext. Serv.
Mississippi State University
Mississippi State, MS 39762
R. Brown, Director
Virgin Islands Coop. Ext. Serv.
University of the Virgin Islands
St. Thomas, USVI 00802
K. Garcia, Director
North Carolina Coop. Ext. Serv.
North Carolina State University
Raleigh, NC 27695-7602
J. F. Ort, Director

vi
Participating State Departments of Agriculture
North Carolina Department of Agriculture and Consumer Services
Agronomic Division
Raleigh, NC 27607-6465
Richard C. Reich, Director
_____________________________________________________________________________________
This bulletin from Regional Project SERA-IEG-6 included researchers from Alabama, Arkansas, Florida,
Georgia, Kentucky, Louisiana, Mississippi, North Carolina, Oklahoma, Puerto Rico, South Carolina,
Tennessee, Texas, Virginia, and the Virgin Islands. It is being electronically published with the approval
of the Directors of the Southern Agricultural Experiment Stations. Under the procedure of coooperative
publications, it becomes in effect, a separate publication for each of the cooperating stations listed.
_____________________________________________________________________________________
Reports of all Southern Region Agricultural Experiment Stations serve people of all ages, socio-economic
levels, race, color, sex, religion, national origin, and the handicapped.
vii
Reference Sufciency Ranges for Plant Analysis
in the Southern Region of the United States
Table of Contents
Preface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ix
Foundation for Practical Application of Plant Analysis — C. R. Campbell and C. O. Plank . . . . 1
Scientic Basis for Plant Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Interpretation Methods Used in Plant Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Sampling Procedures That Enhance Accuracy and Effectiveness . . . . . . . . . . . . . . . . . . . . . . 4
Applications of Plant Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Reference Sufciency Ranges
Field Crops
Canola — C. O. Plank and M. R. Tucker .........................................9
Corn — C. R. Campbell and C. O. Plank ........................................11
Cotton — C. C. Mitchell and W. H. Baker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Grain Sorghum — F. R. Cox and L. Unruh . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Peanut — C. R. Campbell and C. O. Plank . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Rice — P. F. Bell and J. L. Kovar ..............................................25
Small Grain (Barley, Oats, Rye, Wheat) — C. O. Plank and S. J. Donohue . . . . . . . . . . . . . 29
Soybean — W. E. Sabbe, G. M. Lessman and P. F. Bell . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Sugarcane — G. J. Gascho ...................................................35
Tobacco, Burley — C. R. Campbell ............................................39
Tobacco, Flue-Cured — C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Forages & Hay Crops
Alfalfa — C. O. Plank .......................................................45
Coastal Bermuda — C. O. Plank and C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
Tall Fescue — G. M. Lessman and W. O. Thom . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Orchardgrass and Smooth Bromegrass — S. J. Donohue and H. J. Savoy, Jr. ............51
viii
Vegetable Crops
Bell Pepper — E. A. Hanlon and G. J. Hochmuth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Broccoli — E. A. Hanlon and G. J. Hochmuth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
Cantaloupe — R. M. Lippert . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
Carrot — E. A. Hanlon and G. J. Hochmuth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
Cauliower — E. A. Hanlon and G. J. Hochmuth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Celery — E. A. Hanlon and G. J. Hochmuth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
Cucumber — C. R. Campbell .................................................69
Cucumber, Greenhouse — C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
Lettuce, Greenhouse—C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
Muskmelon — E. A. Hanlon and G. J. Hochmuth .................................75
Spinach, Greenhouse — C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Tomato, Greenhouse — C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 79
Tomato, Trellis — C. R. Campbell .............................................81
Vidalia Onion — C. O. Plank .................................................83
Watermelon — R. M. Lippert .................................................87
Turf & Lawn Grasses
Bentgrass — C. R. Campbell and C. O. Plank ....................................91
Bermudagrass (‘Tifgreen’, Tifton-328’) — C. R. Campbell and C. O. Plank . . . . . . . . . . . . 93
Fruit & Nut Crops
Apple — C. O. Plank . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97
Blueberry, Rabbiteye — C. O. Plank and M. R. Tucker .............................99
Grape, Muscadine — C. O. Plank and C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101
Peach — R. M. Lippert and C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103
Pear — C. O. Plank and R. M. Lippert . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
Pecan — C. O. Plank and C. C. Mitchell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 107
Strawberry, Annual Hill Culture — C. R. Campbell and G. S. Miner . . . . . . . . . . . . . . . . . 111
Ornamentals & Flowers
Ornamental Cabbage — C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 115
Poinsettia — C. R. Campbell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 117
Tree Crops
Fraser Fir — C. R. Campbell and L. E. Hinesley .................................121
ix
Preface
Plant analysis has evolved through years of research and experience to become an integral part
of modern crop management. What began as a diagnostic tool to pinpoint nutrient deciencies
in crops exhibiting ymptoms has evolved as a primary tool in nutrient management. Signicant
economic returns are now realized from using plant analysis to guide efcient use of nutrients
in growing healthy crops. As concerns over protecting the environment have gained importance,
plant analysis now plays a critical role in guiding safe use of waste products to grow crops while
ensuring optimum yields and minimizing risk to the environment. During the next decade, plant
analysis will be an integral part of prescription-based, site-specic fertilizer technology.
This bulletin is a reference for information needed to properly interpret plant analysis results
in the Southern United States. Students as well as eld agronomists should nd the bulletin
a valued resource. After extensive reviews of pertinent research, sufciency guidelines are
provided for major crops. The brief outline format facilitates a quick review of the research base
for interpreting analytical results. Where the research base is limited, guidelines are provided
based on surveys and accumulated experience to provide a starting point for further renement.
Special appreciation is expressed to all who contributed to the development of this bulletin
and especially to members of the Southern Extension and Research Activities Group for their
leadership and support of this activity. Appreciation is also expressed to Dr. George Kriz,
Administrative Advisor, for his leadership and sincere support of the work group. Gratitude is
also expressed to the Editorial Committee for their close review of the bulletin.
C. Ray Campbell, Editor
Editorial Committee
C. Ray Campbell, Editor F. R. Cox W. H. Baker
C. O. Plank S. T. Donohue C. C. Mitchell
x
Members of the Southern Extension and Research Activities
Information Exchange Group-6 Soil Test and Plant Analysis 1999
------------------------------------------------------------------------------------------
Administrative Advisor—G. J. Kriz, Associate Director,
Agricultural Experiment Station, North Carolina State University 27695
------------------------------------------------------------------------------------------
Alabama C. C. Mitchell (Rep), J. Adams, B. Hamilton
Arkansas W. E. Sabbe (Rep), W. H. Baker, N. Miller
Florida G. Kidder, J. M. Bartos
Georgia C. O. Plank (Rep), C. W. Jordan
Kentucky W. O. Thom (Rep), D. Kirkland
Louisiana J. Kovar (Rep), P. F. Bell, R. Henderson
Mississippi W. Houston (Rep), K. Crouse
North Carolina F. R. Cox (Rep), M. R. Tucker, C. R. Campbell
Oklahoma E. Allen (Rep), G. V. Johnson
Puerto Rico D. Sotomayor
South Carolina K. Moore
Tennessee G. Lessman (Rep), J. J. Jared, H. J. Savoy
Texas M. Hickey (Rep), S. Perry
Virginia S. J. Donohue (Rep)

1
Foundation for Practical Application of Plant Analysis
C. R. Campbell and C. O. Plank
Modern application of plant analysis has evolved from years of research and
experience with individual crops. In most cases, research was not conducted for
the sole purpose of identifying critical limits or sufciency ranges. These values
were extrapolated from research in which the primary purpose was to develop
response curves for specic fertilizer application and soil test calibration.
Equally important in developing this tool has been experience gained in interpreting plant
results and observing response to fertilizer treatments. Extensive use of plant analysis in solving
problems and managing healthy crops fosters condence in this important management tool.
Scientic Basis for Plant Analysis
Plant analysis is the chemical evaluation of essential element concentrations in plant tissue.
Essential elements include those that are required to complete the life cycle of a plant. The
elements carbon (C), oxygen (O), and hydrogen (H) are supplied by the atmosphere and water
and generally are not considered limiting. Agronomists place most emphases on essential
elements supplied by soil or feeding solutions. Macronutrients — nitrogen (N), phosphorus
(P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) — are required in greatest
quantities. Micronutrients — iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), boron (B),
molybdenum (Mo) and chlorine (Cl) — are required in very small quantities. Toxicities of
micronutrients are equally important and yield limiting as deciencies. Plant analysis is also
effective in diagnosing toxicities of micronutrients. Cobalt (Co) is also essential for symbiotic
N2-xing bacteria associated with legumes.
The interpretation of plant analysis results is based on the scientic principle that healthy plants
contain predictable concentrations of essential elements. A number of researchers have offered
schematics showing the relationship between maximum yield and concentrations of essential
elements (Ulrich and Hills 1967; Brow 1970; Dow and Roberts 1982). Chapman (1967) added
interpretation ranges to these relationships (Fig. 1). Schematics of crop response and nutrient
concentrations are based on general scientic principles and do not account for differences due to
plant part sampled, tissue age, stage of growth, variety, and other factors.
Campbell has further expanded this relationship to include excess and toxic levels of nutrients
along with an interpretation index (Fig. 2). The additional ranges allow agronomists and
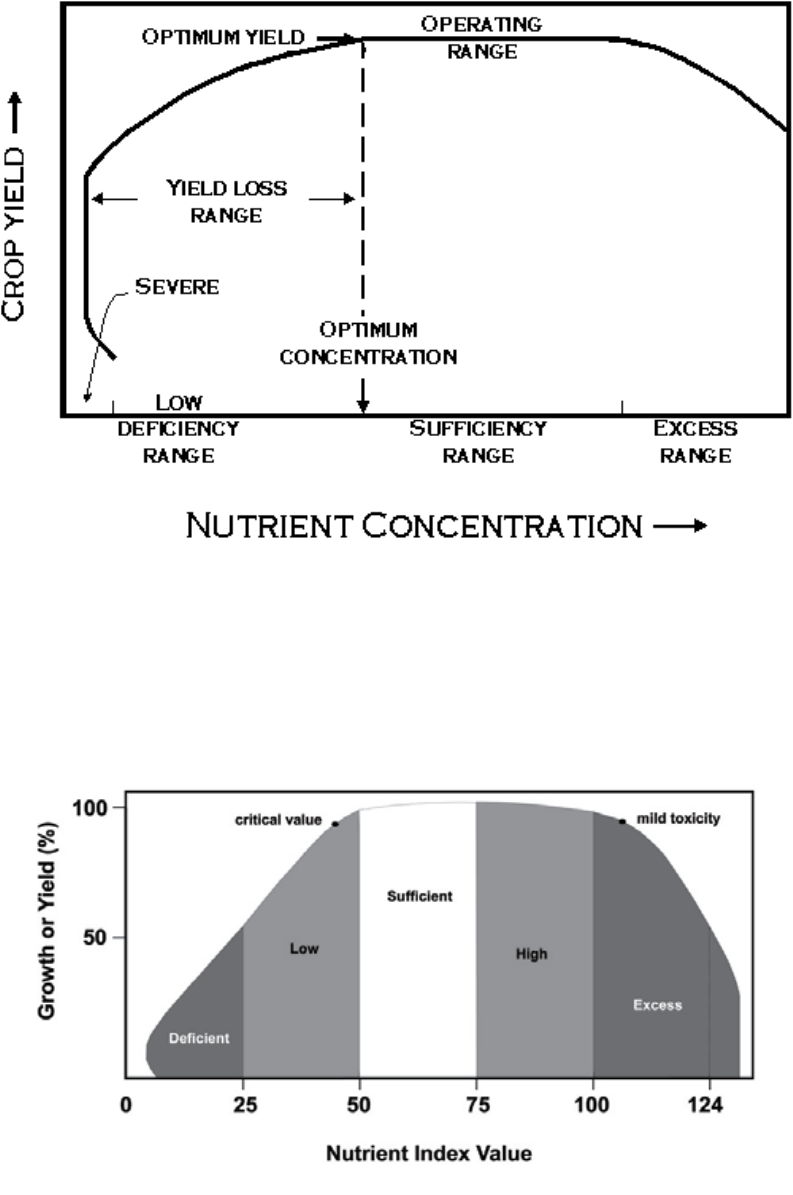
2
Figure 1. Schematic of yield and nutrient concentration (Chapman 1967).
Figure 2. Schematic of yield or growth in response to increasing nutrient concentration
and interpretation.
3
farmers to address excess and toxic levels of elements that may not only inuence growth but
increase risks to the environment. The interpretation index allows practitioners of plant analysis
to become interpreters without extensive training and knowledge of sufciency ranges for
individual elements and crops.
Best indicator samples have been identied for most economically important crops. For crops
receiving greater research support, indicator samples have been identied by stage of growth.
Plant analysis is generally associated with evaluation of leaf samples. In recent years, diagnostic
tests and criteria have been developed for petioles of indicator leaf samples. These tests have
generally served to ne tune the prediction of nitrogen status. Potassium and phosphorus have
also been evaluated in petioles of important crops including cotton, grape, and strawberry.
Nitrate nitrogen or petiole nitrate levels, as they are commonly referred to, indicate the current
status of nitrogen by placing emphases on the mobile form of the element rather than the total
that has been assimilated in the plant.
Interpretation Methods Used in Plant Analysis
There are three major methods of interpreting plant analysis results. They include the use of
critical values, sufciency ranges, and ratios. Most advisory services use sufciency ranges for
primary interpretation. Ratios and DRIS analysis are generally used as secondary and supportive
evaluations.
Critical Values•
Critical values have been dened as the concentration at which there is a 5–10% yield
reduction. The use of critical values for practical interpretation has limited value. It is best
suited to diagnose severe deciencies and has little application in identifying hidden hunger.
Symptoms are generally evident when nutrient concentrations decrease below the critical
value. Critical values play an important role in establishing lower limits of sufciency ranges.
Sufciency Ranges•
Sufciency range interpretation offers signicant advantages over the use of critical values.
First, hidden hunger in the transitional zone can be identied since the beginning of the
sufciency range is clearly above the critical value. Sufciency ranges also have upper limits,
which provide some indication of the concentration at which the element may be in excess.
Ratios•
In simplest form, the use of ratios in the interpretation of plant analysis results involves
the evaluation of two essential elements together recognizing the effect of one element on
the other. The most commonly used ratio is N:S (Nitrogen to Sulfur). The ideal N:S ratio
for most crops is 10–15. As the N:S ratio approaches and exceeds 18, sulfur is limiting in
relation to nitrogen. In reality, the plant does not assimilate nitrogen well because sulfur is
limiting. The N:S ratio can be high when both nitrogen and sulfur concentrations are within
the sufciency ranges for these elements.
4
Other ratios commonly used to support sufciency range interpretation include N:K and
Fe:Mn. Interpretative data bases for these ratios are available for a limited number of crops.
In general, the N:K ratio should be 1.2–2.2. The Fe:Mn ratio should be > 1.
The most complex application of ratios in the interpretation of plant analysis results is DRIS
(Diagnostic Recommendation Integrated System). This technique, which was developed
by Sumner and others (Beauls 1973; Walworth and Sumner 1987), places emphasis on
the relationship among essential elements rather than absolute concentrations. In short,
DRIS ranks the essential elements in their order of limitedness. Theoretically, if the most
limiting element is applied then the second element becomes most limiting. DRIS evaluation
compares ratios of essential elements in the unknown sample to ratios of these elements in
high yielding populations. Modication of DRIS interpretation in recent years to account
for the magnitude of limitedness has signicantly improved this diagnostic tool. Previously,
elements were listed in a descending order of limitedness even when the most limiting
element was not a signicant problem. Normal ratios of high yielding populations are
available for a number of economically important crops.
Sampling Procedures That Enhance Accuracy and Effectiveness
Careful sampling ensures the effectiveness of plant analysis as a diagnostic tool. For major crops,
best indicator samples have been identied by stage of growth. For young seedlings, the entire
plant is sampled 2.5 cm above the soil level. For larger plants, the most recent fully expanded or
mature leaf is the best indicator of nutritional status. As some crops, including corn, approach
owering and fruiting, the best indicator of nutritional status is the leaf adjacent to the uppermost
fruit (earleaf). When unfamiliar with sampling protocol for a specic crop, it is generally
acceptable to select the most recent mature leaf as the best indicator of nutritional status.
A very small amount of plant material is required for a laboratory test (< 1 gram), but reliable
samples must include enough leaves to adequately represent the affected area. For crops with
small leaves (azalea), 25–30 leaves are required for a good sample. Larger leaved crops,
including corn or tobacco, require signicantly fewer leaves for an adequate sample.
Problem Solving•
Diagnostic samples should be taken at the rst indication there may be a problem. Generally,
the earlier in the life cycle of the plant, the more reliable the sample. Samples taken prior
to or at owering are signicantly more reliable than those taken in various stages of
maturity. Comparative samples from good and bad plants allow a high degree of accuracy in
identifying the most limiting element. Matching soil samples taken from the root zones of
plants in each of the sample areas provide more complete information for problem solving.
When symptoms on plants are zonal and the most recent mature leaf appears normal, it
is helpful to sample leaves showing symptoms in addition to the most recent mature leaf.
Knowledge of the accumulation of elements in certain plant parts also helps in selecting
5
additional samples that should be taken when problem solving. For example, bud samples
provide additional conrmation of boron deciency. Likewise, older plant leaves are
important in diagnosing boron toxicity.
Monitoring•
The evaluation of healthy crops in ne tuning nutrient application requires consistent
sampling. Ideally, monitoring samples should be taken the same time of day and from
the same area in the eld each sampling date. If there is wide variability in the eld, it is
desirable to take the sample from a relatively small area. Results can then be evaluated for
that specic area. All other areas in the eld can be compared to the standard sampling area.
Monitoring samples for intensively managed crops, including vegetables in greenhouses
or elds, should be taken no less than every two weeks. Hydroponic crops should be
sampled weekly. Less intensive eld crops, including corn, should be sampled just prior to
sidedressing and at owering. Additional samples are taken as the need arises.
Petiole Sampling•
Petioles for nitrate nitrogen determination should be removed from the most recent mature
leaf or trifoliate. Ideally, petioles should be removed at sampling to avoid further transport
of nitrates. Values generally are lower when petioles are removed at the laboratory.
Petiole nitrate monitoring requires sampling no less than every two weeks during critical
development periods, including owering and fruit development.
Signs of Problems in Sampling•
Chronic deciencies or excesses of certain nutrients may indicate a sampling problem. Since
calcium accumulates in lower leaves as cell walls develop, consistently low levels of this
element when there are no symptoms may indicate the sample is being taken too near the
growing point. Likewise, consistently high calcium and low potassium may indicate the
sample is being taken too far down from the growing point. Comparative sampling of upper
and lower leaves is helpful in identifying the best indicator sample.
Sampling Containers and Laboratory Transport•
Samples should always be shipped to laboratories in a paper container. Plastic containers that
promote respiration and decomposition by disease organisms should never be used. Most
laboratories provide a proper sample container. Samples should be packed loosely so that
drying can begin in transport. Samples can be dried in ovens at 80° C before shipping to save
shipping expense but valuable response time is lost.
Environmental Conditions•
Caution should be exercised when sampling crops damaged by disease, insects, drought and
other factors. Comparative samples of good and bad plants help to neutralize the effects of
some environmental factors. Environmental conditions should always be noted on the sample
information form. Many times plant samples help to eliminate nutrition as a causal agent
when other factors like disease or insect damage are suspected.
6
Applications of Plant Analysis
There are a number of important applications of plant analysis in research and production
agriculture. Plant analysis is very effective in documenting response to nutrient applications.
Leaf concentrations have, therefore, been correlated with yield and soil test values in calibration
work. This data base provides the basis for problem solving and monitoring. Crop requirements
have been well established using plant analysis. Nutrient uptake patterns, accumulation, and
partitioning have been dened for many crops. Fertilizer efciency, depending on placement
and form, have also been effectively studied. Although plant analysis was rst used in
production agriculture to diagnose potential deciencies, it now has developed into an important
management tool in monitoring the nutritional status of healthy crops.
Problem Solving•
Comparative samples from good and bad areas of production elds are very effective in
pinpointing the limiting element(s). Matching soil samples from the root zones of plants in
each of these areas provide additional evidence of the problem and help determine the best
corrective action. Comparative plant and soil samples from areas responding differently
also help to isolate or neutralize the overriding inuence of confounding factors including
moisture, insects, disease, and other sources of injury.
Monitoring•
In recent years, plant analysis has become an integral part of managing healthy crops to
enhance yield and quality while also maximizing efciency and protecting the environment.
As pressure has mounted to dispose of waste products on farm land, plant samples have
provided a means for monitoring these sites to ensure maximum crop performance while
avoiding excess application. Intensively managed vegetable crops with trickle irrigation and
feeding require weekly sampling to guide nutrient management. With interest in precision
agriculture and prescription fertilizer application, monitoring will become even more
important in the future.
References
Beauls ER. 1973. Diagnosis and recommendation integrated system (DRIS). Natal (South
Africa): University of Natal. Soil Science Bulletin No. 1.
Brown JR. 1970. Plant analysis. St. Louis (MO): Missouri Agric Exp Stn. Bulletin SB881.
Dow AI, Roberts S. 1982. Proposal: critical nutrient ranges for crop diagnosis. Agron J 74:401–3.
Russel JS, Bourg CW, Rhoades HF. 1954. Effect of nitrogen fertilizer on the nitrogen,
phosphorus and cation contents of bromegrass. Soil Sci Soc Am Proc 18:292–6.
Ulrich A, Hills FJ. 1967. Principles and practices of plant analysis. In: Soil testing and plant
analysis. Part II. Madison (WI): Soil Science Society of America. (Special publication series; 2).
Walworth JL, Sumner ME. 1987. The diagnosis and recommendation integrated system (DRIS).
In: Stewart BA, editor. Advances in soil science. Volume 6. New York (NY): Springer-Verlag. p
149–88.
7
Reference Sufciency Ranges
— Field Crops —
8

9
Reference Sufciency Ranges — Field Crops
Canola C. O. Plank and M. R. Tucker
Critical Values•
Critical values at 90% relative yield
N P K Ca Mg S Mn Fe B Cu Zn
3.60% 0.37% 2.15% 1.60% 0.10% 0.47% 20 ppm 82 ppm 20 ppm 4 ppm 28 ppm
Sampling Procedures •
Sample the uppermost recently mature leaf blades prior to owering.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
4.00–6.40% 0.42–0.69% 3.50–5.10% 2.10–3.00% 0.15–0.62% 0.65–0.90%
Micronutrients
Fe Mn Zn Cu B
100+ ppm 30–250 ppm 33–49 ppm 5–25 ppm 25–54 ppm
Important Ratios
The calculated N:S ratio should not exceed 16–17 to 1.
DRIS Norms •
DRIS norms have not been reported at this time.
10
Remarks •
The critical values given were calculated from the paper by Haneklaus and Schnug (1991)
and based on 90% relative yield. The critical levels were established using the boundary line
approach of Walworth and others (1986).
The lower end of the sufciency range was calculated at 100% relative yield using the data of
Haneklaus and Schnug (1991) and the upper end of the range was established using the data
reported by Reuter in Reuter and Robinson (1986).
References •
Haneklaus S, Schnug E. 1991. Evaluation of the nutritional status of oilseed rape plants
by leaf analysis. In: Proceedings of the 8th international rapeseed congress; Saskatoon,
Saskatchewan, Canada. p 536–41.
Reuter DJ. 1986. Temperate and sub-tropical crops. In: Reuter DJ, Robinson JB, editors.
Plant analysis: an interpretation manual. Melbourne (Australia): Inkata. p 63–4.
Walworth JL, Letzsch WS, Sumner ME. 1986. Use of boundary lines in establishing
diagnostic norms. Soil Sci Soc Am J 50:123–8.

11
Reference Sufciency Ranges — Field Crops
Corn C. R. Campbell and C. O. Plank
Critical Values•
At Tasseling
Macronutrients
N P K Ca Mg S
3.0% 0.25% 2.0% 0.4% 0.25% 0.12%
Micronutrients
Fe Mn Zn Cu B Mo
15 ppm 15 ppm 15 ppm 5 ppm 10 ppm 0.1 ppm
Sampling Procedures •
Seedling (< 4 inches in height)
Whole plants should be collected by cutting 1 inch above the soil surface. Depending on size,
15 to 20 plants are adequate for a sample.
Early Growth (> 4 inches in height to tasseling)
The most recent mature leaf (MRML) is the best indicator sample. Depending on size, 15 to
20 leaves are adequate for a sample.
Tasseling / Bloom
The earleaf is the best indicator sample. This is the leaf adjacent to the uppermost developing
ear. Fifteen to twenty leaves are adequate for a sample.
Maturity
The earleaf is the best indicator sample. This is the leaf adjacent to the uppermost developing
ear. Fifteen to twenty leaves are adequate for a sample.
Notes for All Samples
Problem-solving samples can be taken at any time during the growing season. Comparative
samples of “good” and “bad” plants or sample areas should be taken according to guidelines
at the stage of growth. Monitoring samples should be taken at lay-by and tasseling (bloom).
Samples should be shipped to the laboratory in paper containers.

12
Sufciency Ranges •
Important Ratios
The N:S ratio should be between 10 and 15 at all growth stages for optimum yields.
Sulfur is limiting at N:S ratios greater than or equal to 18.
Seedling (< 4 inches in height)
Macronutrients
N P K Ca Mg S
4.0–5.0% 0.4–0.6% 3.0–4.0% 0.3–0.8% 0.2–0.6% 0.18–0.5%
Micronutrients
Fe Mn Zn Cu B Mo
40–250 ppm 25–160 ppm 20–60 ppm 6–20 ppm 5–25 ppm 0.1–2.0 ppm
Early Growth (> 4 inches in height to tasseling)
Macronutrients
N P K Ca Mg S
3.0–4.0% 0.3–0.5% 2.0–3.0% 0.25–0.8% 0.15–0.6% 0.15–0.4%
Micronutrients
Fe Mn Zn Cu B Mo
30–250 ppm 20–150 ppm 20–70 ppm 5–25 ppm 5–25 ppm 0.1–2.0 ppm
Tasseling / Bloom
Macronutrients
N P K Ca Mg S
2.8–4.0% 0.25–0.5% 1.8–3.0% 0.25–0.8% 0.15–0.6% 0.15–0.6%
Micronutrients
Fe Mn Zn Cu B Mo
30–250 ppm 15–150 ppm 20–70 ppm 5–25 ppm 5–25 ppm 0.1–2.0 ppm
Maturity
Macronutrients
N P K Ca Mg S
2.5–3.5% 0.25–0.4% 1.6–2.5% 0.2–0.8% 0.12–0.5% 0.12–0.4%
Micronutrients
Fe Mn Zn Cu B Mo
30–250 ppm 15–150 ppm 16–50 ppm 4–20 ppm 3–20 ppm 0.1–2.0 ppm

13
Reference Sufciency Ranges — Field Crops
DRIS Norms •
DRIS norms, based on a high-yielding subpopulation, have been provided by Elwali and
others (1985).
Parameter No. Mean SD Parameter No. Mean SD
N/P ‡ 1909 9.035 2.136 10 N/Zn 1526 11.797 4.459
N/K 1908 1.463 0.426 Zn/10 P 1527 0.883 0.420
P/K 1909 0.169 0.054 Zn/10 K 1526 0.140 0.068
Ca/N 1553 0.160 0.057 10 Ca/Zn 1524 1.919 1.087
Ca/P 1554 1.447 0.612 10 Mg/Zn 1527 0.830 0.504
Ca/K 1553 0.237 0.122 10 S/Zn 760 0.952 0.365
Mg/N 1556 0.071 0.029 Fe/Zn 1268 4.464 1.837
Mg/P 1557 0.639 0.330 Mn/Zn 1520 1.716 1.175
Mg/K 1556 0.104 0.063 Cu/10 N 1401 0.031 0.013
Mg/Ca 1554 0.465 0.182 Cu/10 P 1402 0.277 0.140
S/N 788 0.084 0.019 Cu/10 K 1401 0.045 0.022
S/P 788 0.703 0.225 10 Ca/Cu 1402 6.022 3.511
S/K 787 0.114 0.029 10 Mg/Cu 1402 2.768 1.935
Ca/S 785 1.978 0.893 Cu/10 S 664 0.375 0.211
S/Mg 788 1.195 0.395 Cu/Fe 1236 0.079 0.036
Fe/10 N 1297 0.394 0.097 Cu/Mn 1395 0.260 0.174
Fe/10 P 1298 3.588 1.177 Cu/Zn 1372
0.356 0.200
Fe/10 K 1297 0.568 0.201 B/10 N 402 0.024 0.012
10 Ca/Fe 1298 0.410 0.189 B/10 P 403 0.269 0.135
10 Mg/Fe 1298 0.190 0.098 B/10 K 402 0.043 0.033
Fe/10 S 687 4.868 1.419 B/10 Ca 403 0.153 0.076
Mn/10 N 1459 0.151 0.087 B/10 Mg 403 0.335 0.152
Mn/10 P 1550 1.416 1.063 10 S/B 112 3.185 1.039
Mn/10 K 1549 0.218 0.140 B/Fe 389 0.068 0.036
Mn/10 Ca 1547 1.048 0.676 B/Mn 399 0.173 0.150
Mn/10 Mg 1550 2.485 1.780 B/Zn 410 0.265 0.134
10 S/Mn 782 0.648 0.351 B/Cu 401 0.950 0.620
Mn/Fe 1293 0.405 0.249
‡ Nutrient concentrations are expressed in g/kg for N, P, K, Ca, Mg, and S and in
mg/kg for Fe, Mn, Zn, Cu, and B. The data presented are number of observations
(No.), means, and standard deviations (SD) of DRIS reference parameters in the
subpopulation yielding > 10.0 Mg of grain per hectare.
14
Remarks •
Sufciency ranges are based on available literature and experience interpreting plant samples.
DRIS should be used to support sufciency range interpretation and identify the most
limiting element or order of impact on growth.
Results are less reliable as corn approaches maturity. Comparative “good” and “bad” samples
should be used when sampling during various stages of maturity.
References •
Elwali AMO, Gascho GJ, Sumner ME. 1985. DRIS norms for 11 nutrients in corn leaves.
Agron J 77:506–8.
Jones JB Jr, Eck HV, Voss R. 1990. Plant analysis as an aid in fertilizing corn and grain
sorghum. In: Westerman RL, editor. Soil testing and plant analysis. 3rd ed. Madison (WI):
Soil Society of America, Inc. p 521–47. (SSSA book series; 3).
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. p 21–8.

15
Reference Sufciency Ranges — Field Crops
Cotton C. C. Mitchell and W. H. Baker
Sufciency Levels and Critical Values•
Sufciency ranges for cotton have often been used based upon observations and ranges of
analyses of plant tissue from healthy or normal cotton crops. For this reason, ranges may
be broad and too inclusive. Therefore, use of a sufciency range for cotton and the implied
critical concentration (lower end of sufciency range) of a nutrient for deciencies or
toxicities are not absolute.
Sampling Procedures •
Petiole analysis
Sample petioles from the most recently matured leaf on the vegetative stem at intervals
beginning the week before rst bloom and continuing for 7 or 8 weeks after bloom. Samples
should be taken at weekly intervals and compared for the results to be meaningful. Interpret
petiole analysis for NO3-N, total P, and total K only. Nitrate analysis is the most meaningful
and the primary reason for sampling.
Leaf blade at early bloom
Sample the uppermost, mature cotton leaf blade on the vegetative stem. Discard the petiole.
(Note: some research has included both leaf blade and petiole.] This is usually the 3rd to 5th
leaf from the terminal. Sample during the period of one week before to one week after rst
bloom.
Sufciency Ranges •
Petioles
The petioles from the most recently matured leaf on the vegetative stem at intervals beginnin
“Arkansas Intepretation” may be more appropriate for loess and other ne-textured soils of
the mid-South whereas the “Georgia Interpretation” was developed for the coarser textured
soils of the Atlantic and Gulf Coastal Plain.

16
“Arkansas” Interpretation (Benton and others 1979)
Time of sampling Nitrate nitrogen (ppm) Phosphorus (ppm)
Week of bloom 10,000–35,000 >800
Bloom + 1 week 9,000–30,000 *
Bloom + 2 weeks 7,000–25,000 *
Bloom + 3 weeks 5,000–20,000 *
Bloom + 4 weeks 3,000–13,000 *
Bloom + 5 weeks 2,000–8,000
Bloom + 6 weeks 1,000–5,000
Bloom + 7 weeks 0–5,000
Bloom + 8 weeks 0–5,000
* A decrease in P concentration of more than 300 ppm from the previous week usually
indicates moisture stress
“Georgia” Interpretation (Lutrick and others 1986; Plank, personal communication)
Time of sampling Nitrate nitrogen (ppm) Phosphorus (ppm)
Week before rst bloom 7,000–13,000 >800
Week of bloom 4,500–12,500 >800
Bloom + 1 week 3,500–11,000 *
Bloom + 2 weeks 2,500–9,500 *
Bloom + 3 weeks 1,500–7,500 *
Bloom + 4 weeks 1,000–7,000 *
Bloom + 5 weeks 1,000–6,000 *
Bloom + 6 weeks 500–4,000
Bloom + 7 weeks 500–4,000
Bloom + 8 weeks 500–4,000
* A decrease in P concentration of more than 300 ppm from the previous week usually
indicates moisture stress
“California” Petiole K Interpretation (Bassett and MacKenzie 1976)
Time of sampling % Potassium (K)
Week of rst bloom 4.0–5.5
Bloom + 4 weeks 3.0–4.0
Bloom + 6 weeks 1.5–2.5
Bloom + 8 weeks 1.0–2.0

17
Reference Sufciency Ranges — Field Crops
Youngest, Mature Leaf Blade
The following sufciency ranges were compiled from several sources (Anderson and others
1971; Hodges and Hadden 1992; Mullins and Burmester 1990, 1992, 1993; Plank 1988;
Reeves and Mullins 1993; Sabbe and Mackenzie 1973; Sabbe and others 1972).
Macronutrients (%)
N P K Ca Mg S
early bloom 3.0–4.5 0.2–0.65 1.5–3.0 2.0–3.5 0.3–0.9 0.25–0.8
late bloom / maturity 3.0–4.5 0.15–0.6 0.75–2.5 2.0–4.0 0.3–0.9 0.3–0.9
Micronutrients (ppm)
Fe Mn Zn Cu B
early bloom 50–250 25–350 20–200 5–25 20–80
late bloom / maturity 50–300 10–400 50–300 15–200
References•
Anderson OE, Perkins HF, Carter RL, Jones JB Jr. 1971. Plant nutrient survey of selected
plants and soils of Georgia. Athens (GA): Georgia Agricultural Experiment Station. Research
Report 102.
Bassett DM, MacKenzie AJ. 1976. Plant analysis as a guide to cotton fertilization. In:
Reisenauer HM, editor. Soil and plant-tissue testing in California. Davis (CA): University of
California Cooperative Extension Service. p 16–7.
Benton ME, Maples R, May RD, Miley WN, Sabbe WE. 1979. A computerized system
for cotton nitrate monitoring with program listings and descriptions. Fayetteville (AR):
University of Arkansas Agricultural Experiment Station. Report Series 244.
Hodges SC, Hadden J. 1992. Late season soil and plant nutrient status in Georgia cotton
soils. In: Proceedings 1992 beltwide cotton conferences. Memphis (TN): National Cotton
Council. p 1126–7.
Lutrick MC, Peacock HA, Cornell JA. 1986. Nitrate monitoring for cotton lint production on
a Typic Paleudult. Agron J 78:1041–6.
Maples R, Keogh JG, Sabbe WE. 1977. Nitrate monitoring for cotton production in Loring-
Calloway silt loam. Fayetteville (AR): University of Arkansas Agricultural Experiment
Station. Bulletin 825.
Miley WN, Bonner CM, Maples R. 1988. Update on cotton petiole testing. Fayetteville (AR):
University of Arkansas Cooperative Extension Service. Cotton Comments 6-88.
18
Miley WN, Maples R. 1988. Cotton nitrate monitoring in Arkansas. Fayetteville (AR):
University of Arkansas Cooperative Extension Service. Cotton Comments 2-88.
Mitchell CC, Pate G, Burmester CH, Edmisten KL, Gazaway W. 1992. Fertility status of
Alabama cotton soils. In: Proceedings 1992 beltwide cotton conferences. Memphis (TN):
National Cotton Council. p 1120–5.
Mullins GL, Burmester CH. 1990. Dry matter, nitrogen, phosphorus, and potassium
accumulation by four cotton varieties. Agron J 82:729–36.
Mullins GL, Burmester CH. 1992. Uptake of calcium and magnesium by cotton grown under
dryland conditions. Agron J 84:564–9.
Mullins GL, Burmester CH. 1993. Accumulation of copper, iron, manganese and zinc by four
cotton cultivars. Field Crops Res 32:129–40.
Plank CO. 1988. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service.
Reeves DW, Mullins GL. 1993. Subsoiling and K placement: effects on cotton water
relations. In: Proceedings 1993 beltwide cotton conferences. Memphis (TN): National Cotton
Council. p 1322–5.
Sabbe WE, Keogh JL, Maples R, Hileman LH. 1972. Nutrient analysis of Arkansas cotton
and soybean leaf tissue. Arkansas Farm Res 21:2.
Sabbe WE, MacKenzie AJ. 1973. Plant analysis as an aid to cotton fertilization. In: Walsh
LM, Beaton JD, editors. Soil testing and plant analysis. Madison (WI): Soil Science Society
of America, Inc. p 299–313.

19
Reference Sufciency Ranges — Field Crops
Grain Sorghum F. R. Cox and L. Unruh
Critical Values•
There are critical values for both deciency and toxicity that presumably set the levels
at which below the former and above the latter there would be a yield depression. There
are numerous observations on the critical level for deciency where the break between
that and sufciency is usually fairly sharp, but very few on toxicity where there is a more
gradual transition from adequate to excess. Both of these points are not exact, but vary with
environmental conditions, varieties, etc. The critical level for deciency sets the lower limit
of the sufciency range as will be used in the tables that follow, but it should be remembered
that this value may not be exact; it can vary 25% or more with changes in extraneous
conditions. In that there is little data for setting the critical level for toxicity, the sufciency
range is usually between the critical level for deciency and a “high” value, which really has
no particular meaning but may be around the maximum concentration ordinarily observed.
Any known or estimated critical levels for toxicity will be covered in the “Remarks” section.
Sampling Procedures (Jones and others 1971) •
Seedling Stage (< 4 cm tall)
Sample whole aboveground portion of plant.
Vegetative or Prior to Heading
Sample entire, fully developed leaf below the whorl.
Flowering or at Heading
Sample second leaf from the top of the plant. This is the recommended sampling procedure
when determining the nutrient status of the treatments, and yield.
Grain Filling
Sample second leaf from the top of the plant.

20
Sufciency Ranges •
Seedling
Macronutrients
N P K Ca Mg S
3.9– % 0.2–0.5% 2.0– % 0.3–0.6% 0.25–0.6% 0.24+ %
Micronutrients
Fe Mn Zn Cu B
75–400 ppm 13–200 ppm 12–150 ppm 4–20 ppm 3–30 ppm
Vegetative
Macronutrients
N P K Ca Mg S
3.0–4.0% 0.2–0.4% 2.0– % 0.3–0.6% 0.2–0.5%
Micronutrients
Fe Mn Zn Cu B
75–200 ppm 8–100 ppm 12–100 ppm 2–15 ppm 1–10 ppm
Flowering
Macronutrients
N P K Ca Mg S
2.5–4.0% 0.20–0.35% 1.4– % 0.3–0.6% 0.2–0.5%
Micronutrients
Fe Mn Zn Cu B
65–100 ppm 8–100 ppm 12–100 ppm 2–7 ppm 1–10 ppm
Grain Filling
Macronutrients
N P K Ca Mg S
2.4–4.0% 0.2–0.3% 1.4– % 0.3–0.6% 0.1–0.5%
Micronutrients
Fe Mn Zn Cu B
40–80 ppm 8–100 ppm 12–100 ppm 1–5 ppm 1–6 ppm

21
Reference Sufciency Ranges — Field Crops
DRIS Norms •
Chemical analyses for the high-yielding subpopulation of sorghum crops and resulting
norms selected for DRIS indices (Arogun 1978) §
Element / Parameter Mean (g/kg) CV (%)
N 30.3 17
P 3.4 15
K 13.1 11
Ca 4.4 20
Mg 2.4 24
P/N 0.112 19
N/K 2.355 23
P/K 0.259 21
N/Ca 7.200 30
P/Ca 0.759 31
K/Ca 3.080 24
Mn/N 0.079 26
P/Mg 1.518 45
Mg/K 0.183 26
Mg/Ca 0.553 30
§ Means and coefcients of variation in the subpopulation (135 of 907 crops) yielding
>7.1 Mg of grain ha-1.
Remarks •
Some recorded toxicity levels at the seedling stage are: Mn >500 ppm, Zn >300 ppm, Na >30
ppm, and Cl >0.2%.
References •
Agarwala SC, Sharma CP. 1979. Recognizing micronutrient disorders of crop plants on the
basis of visible symptoms and plant analyses. Lucknow (India): Lucknow University.
Arogun JO. 1978. Application of the DRIS system to sorghum and millet [MSc thesis].
Madison (WI): University of Wisconsin.
Clark RB. 1993. Sorghum. In: Bennett WF, editor. Nutrient deciencies & toxicities in crop
plants. St. Paul (MN): American Phytopathological Society. p 21–6.
22
de Boer GJ, Reisenauer HM. 1973. DTPA as an extractant of available soil iron. Commun
Soil Sci Plant Anal 4:121–8.
Francois LE, Donovan T, Mass EV. 1984. Salinity effects on seed yield, growth and
germination of grain sorghum. Agron J 76:741–4.
Grundon NJ, Edwards DG, Takkar PN, Asher CJ, Clark RB. 1987. Nutritional disorders
of grain sorghum. Canberra (Australia): Australian Centre for International Agricultural
Research.
Jones JB Jr, Eck HV, Voss R. 1990. Plant analysis as a aid in fertilizing corn and grain
sorghum. In: Westerman RL, editor. Soil testing and plant analysis. 3rd ed. Madison (WI):
Soil Science Society of America. p 521–47.
Jones JB Jr, Large RL, Pfeiderer DB, Klosky HS. 1971. How to properly sample for a plant
analysis. Crops Soils 23:114–20.
Lockman RB. 1972a. Mineral composition of grain sorghum plant samples. Part I,
Comparative analysis with corn at various stages of growth and under different environments.
Commun Soil Sci Plant Anal 3:271–82.
Lockman RB. 1972b. Mineral composition of grain sorghum plant samples. Part III,
Suggested nutrient sufciency limits at various stages of growth. Commun Soil Sci Plant
Anal 3:295–304.
Ohki K. 1975. Manganese supply, growth and micronutrient concentration in grain sorghum.
Agron J 67:30–2.
Ohki K. 1984. Zinc nutrition related to critical deciency and toxicity levels for sorghum.
Agron J 76:253–6.
Reuter DJ. 1986. Temperate and sub-tropical crops. In: Reuter DJ, Robinson JB, editors.
Plant analysis: an interpretation manual. Melbourne (Australia): Inkata. p 39–99.
Weir RG. 1983. Tissue analysis for pastures and eld crops. Canberra (Australia): New South
Wales Department of Agriculture. Advisory Note No. 11/83.

23
Reference Sufciency Ranges — Field Crops
Peanut C. R. Campbell and C. O. Plank
Critical Values•
None reported.
Sampling Procedures •
All Growth Stages
Sample whole aboveground portion of plant.
Problem-solving Samples
Sample entire, fully developed leaf below the whorl.
Monitoring Samples
Sample second leaf from the top of the plant. This is the recommended sampling procedure
when determining the nutrient status of the treatments, and yield.
Sufciency Ranges •
All Growth Stages
Macronutrients
N P K Ca Mg S
3.5–4.5 % 0.2–0.5% 1.7–3.0% 0.5–2.0% 0.3–0.8% 0.2–0.35%
Micronutrients
Fe Mn Zn Cu B Mo
50–250 ppm 20–350 ppm 20–60 ppm 5–20 ppm 20–60 ppm 0.1–5.0 ppm
Important Ratios
Ca:Zn ratios less than 45–50 indicate zinc toxicity.
24
DRIS Norms •
DRIS norms have not been reported for peanut.
Remarks •
Sufciency ranges are based on available literature and extensive experience interpreting
plant samples.
Zinc toxicity is a signicant problem and occurs when zinc concentration approaches 200
ppm. Zinc toxicity is usually associated with low pH and extensive municipal or animal
waste application.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. p 21–8.

25
Reference Sufciency Ranges — Field Crops
Rice Paul F. Bell and John L. Kovar
Critical Values•
A critical value is dened as the concentration of an essential element at which there is a
5–10% reduction in growth or yield.
Sampling Procedures •
Mid-tillering
Leaf samples should be taken from the youngest, fully developed leaves. About twenty leaves
should be collected. Critical values for sulfur (S) were developed from analysis of whole
plant (above-ground) samples.
Panicle Initiation
Leaf samples should be taken from the youngest, fully developed leaves. These are the
Y-leaves. About twenty leaves should be collected. The panicle should be at least 2 mm in
length.
Sufciency Ranges •
Mid-tillering
Macronutrients
N P K Ca Mg S
2.8–3.6% 0.14–0.27% 1.5–2.7% 0.16–0.39% 0.12–0.21% 0.17+ %
Micronutrients
Fe Mn Zn Cu B
90–190 ppm 40–740 ppm 20–160 ppm 6–25 ppm 5–25 ppm
Important Ratios
For adequate N and S, the N/S ratio should be < 10, with N > 1.6% and S > 0.15%.

26
Panicle Initiation
Macronutrients
N P K Ca Mg S
3.0–3.4% 0.18–0.29% 1.5–2.7% 0.19–0.39% 0.15–0.39% 0.15+ %
Micronutrients
Fe Mn Zn Cu B
70–190 ppm 40–800 ppm 20–160 ppm 6–25 ppm 6–15 ppm
Important Ratios
For adequate N and S, the N/S ratio should be < 10, with N > 1.6% and S > 0.15%.
DRIS Norms •
Nutrient Ratio Mean CV (%) Nutrient Ratio Mean CV (%)
N/P 9.8174 13.2 10 P/Fe 0.6195 80.7
N/K 1.19847 32.5 K/Mg 20.0648 21.7
N/Ca 6.7736 33.5 K/S 16.0629 66.5
N/S 17.2864 53.3 K/Cu 6.4452 18.7
N/Mg 19.7246 18.8 K/Fe 0.6012 91.7
10 N/Cu 6.3309 15.0 Ca/S 3.00039 82.8
P/K 0.12042 23.2 10 Ca/Fe 0.873 59.2
P/Ca 0.71713 28.2 Mg/S 0.94908 60.5
P/Mg 2.12043 17.8 Mg/Cu 0.3302 20.7
P/S 1.80124 56.4 10 Mg/Fe 0.298 85.6
10 P/Cu 6.811 13.8 Fe/Mn 0.15069 35.1
Remarks •
The information presented in the section is based on the published research cited in the
reference list. DRIS norms were developed from a database of eastern Arkansas rice tissue
analyses and yields (Counce and Wells 1986). A reliable sufciency range for S diagnosis
was not available. Rice varieties differ in both their requirement for N and leaf N critical
values (Brandon and Wells 1986).
In addition to sufciency ranges, nutrient and other ion toxicities also have been reported.
Aluminum (Al) toxicity is likely if whole plant Al is >300 ppm (Tanaka and Yoshida 1970).
Research (Baker and others 1976) has shown that rice is sensitive to soil arsenic (As). The
critical level in shoots ranges from 20–100 ppm. In roots, the critical level is 1000 ppm.
27
Reference Sufciency Ranges — Field Crops
Paddy rice is more susceptible to As toxicity due to the presence of more readily absorbed
arsenite (As III). In some cases, ferrous iron (Fe II) may also pose a toxicity problem.
Toxicity is possible in rice if chloride (Cl) reaches >10,000 ppm and nitrate >1600 ppm
(Helms 1994). Leaf concentrations of manganese (Mn) in the range 4000–8000 ppm are toxic
to rice (Adriano 1986). Molybdenum (Mo) toxicity is very rare, but an approximate value
would be >100 ppm for leaves from grass species such as rice (Jones 1991). In Louisiana,
sodic injury can occur when leaf Na in pre-boot-stage rice exceeds 2000 ppm. Zinc (Zn)
toxicity was reported by Chino (1981) when rice shoots contained 100–300 ppm and rice
roots contained 500–1000 ppm.
With respect to deciencies, rice and other cereal grasses are not sensitive to low Mo. For
whole plants at boot stage, 0.09–0.18 ppm are considered sufcient. Deciency of silicon
(Si) may occur when Si is <5% in straw sampled at maturity (Tanaka and Yoshida 1970).
References •
Adriano DC. 1986. Trace elements in the terrestrial environment. New York: Springer-Verlag.
Baker RS, Barrentine WL, Bowman DH, Hawthorne WL, Pettiet JV. 1976. Crop response
and arsenic uptake following soil incorporation of MSMA. Weed Sci 24:322–6.
Brandon DM, Wells BR. 1986. Improving nitrogen fertilization in mechanized rice culture.
Fert Res 9:161–70.
Chino M. 1981. Metal stress in rice plants. In: Kitagishi K, Yamane I, editors. Heavy metal
pollution in soils of Japan. Tokyo: Japan Science Society Press. p 65–80.
Counce PA, Wells BR. 1986. Rice Y-leaf nutrient analyses and midseason, foliar fertilization.
Commun Soil Sci Plant Anal 17:1071–87.
Helms RS. 1994. Rice production handbook. Little Rock: University of Arkansas
Cooperative Extension Service. Publication MP 192-2M-4-94R.
Jones JB Jr. 1991. Plant tissue analysis in micronutrients. In: Mortvedt JJ, Cox FR, Shuman
LM, Welch RM, editors. Micronutrients in agriculture. Madison (WI): American Society of
Agronomy. p 477–522.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. p 130.
Jones US. 1982. Fertilizers and soil fertility. Reston (VA): Reston Publishing Co.
28
Sedberry JE Jr, Amacher MC, Bligh DP, Curtis OD. 1987. Plant-tissue analysis as a
diagnostic aid in crop production. Baton Rouge: Louisiana Agricultural Experiment Station.
Bulletin No. 783.
Suzuki A. 1978. Sulfur nutrition and diagnosis of sulfur deciency of rice plants. JARQ
12:7–11.
Tanaka A, Yoshida S. 1970. Nutritional disorders of the rice plant in Asia. Manila
(Philippines): International Rice Research Institute. Technical Bulletin 10.
Yoshida S, Chaudhry MR. 1979. Sulfur nutrition of rice. Soil Sci Plant Nutr 25:121–34.

29
Reference Sufciency Ranges — Field Crops
Small Grain
—Barley, Oats,
Rye, Wheat C. O. Plank and S. J. Donohue
Critical Values •
The values given here are best estimates based on extensive experience. They apply to all
samples and growth stages.
Macronutrients
N P K Ca Mg S
3.0% 0.15% 2.0% 0.15% 0.10% 0.10%
Micronutrients
Fe Mn Zn Cu B Mo
25 ppm 15 ppm 15 ppm 3 ppm 1 ppm 0.05 ppm
Sampling Procedures •
Seedling to Tillering
Whole plants should be collected by cutting 1 inch above the soil surface. Samples can
be taken by grasping existing growth at a given site and cutting at the recommended level
above the soil with a small knife. Dead leaves should be avoided as much as possible. After
collecting subsamples from several locations in a eld, clippings should be combined for a
representative sample.
Jointing to Flag Leaf Emergence
Break the top two to three leaves (growing point) from representative plants in several
locations of the eld. Combine for a representative sample. Stems should be included.
Flag Leaf to Maturity
Flag leaves from representative plants in the eld should be collected randomly. A minimum
of 15 to 20 leaves should be collected from a given eld or area.

30
Problem-solving Samples
These samples can be taken at any time during the growing season. Comparative samples
from “good” and “bad” areas should be taken according to guidelines at the stage of growth.
Monitoring Samples
These samples should be taken at full tillering (Zadoks 30; Feekes 5) to predict nutritional
status and additional nitrogen required to optimize yield. Final monitoring samples should be
taken at ag leaf emergence (Zadoks 45; Feekes 10) to evaluate nutrient program.
Sufciency Ranges •
Important Ratios
The N:S ratio should be between 10 and 15 for optimum yields. N:S ratios greater than
or equal to 18 indicate that sulfur is limiting in relation to nitrogen.
Seedling to Tillering; Jointing to Flag Leaf Emergence
Macronutrients
N P K Ca Mg S
4.0–5.0% 0.2–0.5% 2.5–5.0% 0.2–1.0% 0.14–1.0% 0.15–0.65%
Micronutrients
Fe Mn Zn Cu B Mo
30–200 ppm 20–150 ppm 18–70 ppm 4.5–15 ppm 1.5–4 ppm 0.1–2.0 ppm
Flag Leaf Maturity
Macronutrients
N P K Ca Mg S
4.0–5.0% 0.2–0.5% 2.0–4.0% 0.2–1.0% 0.14–1.0% 0.15–0.65%
Micronutrients
Fe Mn Zn Cu B Mo
30–200 ppm 20–150 ppm 18–70 ppm 4.5–15 ppm 1.5–4.0 ppm 0.1–2.0 ppm
DRIS Norms •
DRIS norms for small grains have not been reported.
31
Reference Sufciency Ranges — Field Crops
Remarks •
Sufciency ranges are based on available literature and experience interpreting plant samples.
Results are less reliable as crop approaches maturity. Comparative “good” and “bad” samples
should be used when sampling at various stages of maturity.
Sufciency ranges can generally be applied for wheat, oats, rye, and barley although most of
the research has been done on wheat.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. p 37–8.
Westfall DG, Whitney DA, Brandon DM. 1990. Plant analysis as an aid in fertilizing small
grains. In: Westerman RL, editor. Soil testing and plant analysis. 3rd ed. Madison (WI): Soil
Society of America, Inc. p 495–519. (SSSA book series; 3).
32

33
Reference Sufciency Ranges — Field Crops
Soybean W. E. Sabbe, G. M. Lessman and P. F. Bell
Critical Values •
Presently, critical values for the R2 stage are 0.30% P, 1.50% K, 17 ppm Mn and 21
ppm Zn. These values are included in a manuscript submitted for publication (personal
communication P. Bell).
Sampling Procedures •
Early Growth and Flowering
The most recently mature leaf blades are collected for subsequent analysis.
Sufciency Ranges •
Early Growth
Macronutrients
N P K Ca Mg S
3.5–5.5% 0.30–0.60% 1.7–2.5% 1.1–2.2%
0.30–0.60%
Flowering
Macronutrients
N P K Ca Mg S
3.25–5.0% 0.30–0.60% 1.5–2.25% 0.8–1.4% 0.25–0.70% 0.25–0.60%
Micronutrients
Fe Mn Zn Cu B
25–300 ppm 17–100 ppm 21–80 ppm 4–30 ppm 20–60 ppm
DRIS Norms •
DRIS norms and indices are currently under study.
Remarks •
The sufciency ranges and critical levels were the result of the chosen references. With three
exceptions (Anderson and others; Dombeck and Sabbe; Sabbe and others unpublished data),
all references were research based with most being fertilizer amendment studies. No data on
sufciency ranges for seedling data are presented as that aspect is not well researched.
34
References •
Anderson OE, Carter RL, Perkins HF, Jones JB Jr. 1971. Plant nutrient survey of selected
plants and soils of Georgia. Athens (GA): University of Georgia Agricultural Experiment
Station. Research Report 102.
Bell PF, Hallmark WB, Sabbe WE, Dombek DG. 1995. Diagnosing nutrient deciencies in
soybean, using M-DRIS and critical nutrient level procedures. Agron J 87:859–65.
Beverly RB, Sumner ME, Letzech WS, Plank CO. 1986. Foliar diagnosis of soybean by
DRIS. Commun Soil Sci Plant Anal 17:237–56.
Bhangoo MS, Albritton DJ. 1972. Effect of fertilizer nitrogen, phosphorus and potassium on
yield and nutrient content of Lee soybean. Agron J 64:743–6.
Hallmark WB, Beverly RB, Sumner ME, De Mooy CJ, Morris HF, Pesek J, Fontenot JD. 1990.
Soybean phosphorus and potassium evaluation by three MDRIS bases. Agron J 82:323–8.
Hawes, RL, Sims JL, Wells KL. 1976. Molybdenum concentration of certain crop species as
inuenced by previous applications of molybdenum fertilizer. Agron J 68:217–8.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 130 p.
Keogh JL, Maples R. 1974. Soybean response to phosphorus, potassium and sulfur in eastern
Arkansas. Fayetteville (AR): University of Arkansas Agricultural Experiment Station. Report
Series 215.
Keogh JL, Maples R. 1976. Response of soybean grown on silt loam soils to direct and
residual phosphorus and potassium. Fayetteville (AR): University of Arkansas Agricultural
Experiment Station. Report Series 225.
Keogh JL, Sabbe WE, Caviness CE. 1972. Nutrient concentration of selected soybean
cultivars. Commun Soil Sci Plant Anal 3:29–35.
Keogh, JL, Sabbe WE, Caviness CE. 1977. Leaf nutrient concentration in selected soybean
cultivars as affected by fertilization, stage of growth and year. Fayetteville (AR): University
of Arkansas Agricultural Experiment Station. Report Series 234.
Sabbe WE, Keogh JL, Maples R, Hileman LH. 1972. Nutrient analysis of Arkansas cotton
and soybean leaf tissue. Ark Farm Res 21:2.
Sedberry JE Jr, Amacher MC, Bligh DP, Curtis OD. 1987. Plant-tissue analysis as a
diagnostic aid in crop production. Baton Rouge (LA): Louisiana Agricultural Experiment
Station. Bulletin 783.
Small HG Jr, Ohlrogge AJ. 1973. Plant analysis as an aid in fertilizing soybeans and peanuts.
In: Walsh LM, Beaton JD, editors. Soil testing and plant analysis. Madison (WI): Soil
Science Society of America. p 315–28.
Sumner ME. 1977. Preliminary N, P, and K foliar diagnostic norms for soybeans. Agron J
69:226–30.

35
Reference Sufciency Ranges — Field Crops
Sugarcane G. J. Gascho
Critical Values•
Critical values in the literature vary with plant part sampled, plant age, variety and with the
time of the day sampled. For the top-visible dewlap leaf during the “Grand Growth” period,
the following critical levels have been published (Evans 1956; Gascho and Elwali 1978).
Macronutrients
N P K Ca Mg S
1.80% 0.19% 0.90% 0.20% 0.12%
Micronutrients
Fe Mn Zn Cu B
5 ppm 25 ppm 15 ppm 3 ppm 4 ppm
Sampling Procedures •
Several systems have evolved in tissue sampling of sugarcane. Much is sampled just prior
to the “Grand Growth” period. However, that period is often difcult to determine as the
harvest varies from 9 months to up to 4 years from planting or ratooning. Therefore, various
sampling practices are conducted in different areas.
A common practice is to use the leaf-blade lamina (midrib removed). The leaf selected is
often the third (3) from the top of the plant. Some agronomists identify this leaf-blade by
nding the uppermost leaf which has a distinct collar on the stalk termed “top-visible dewlap
leaf.”
A system of “crop logging” developed in Hawaii (Clements 1980) utilizes the leaf sheaths
from the 3rd to the 6th leaves from the top of the plant for P, K, Ca and Mg and the lamina
of the leaf blades from the same leaves for N. Another sampling procedure utilizes stalk
internodes number 8 to 10 from the base of the stalk (Hawaiian Sugar Planters Association).
Care must be exercised to standardize the time of day that samples are collected. Thein
and Gascho (1980) found that concentrations of N, P, Ca and Mg in leaf samples decreased
signicantly during the day (Table 1). Early morning sampling is preferred (Clements 1980).

36
Table 1. Mean tissue nutrient concentrations in sugarcane TVD leaf blade laminas as a function
of time of day § †
Time of Day
Plant Tissue Nutrients
%N %P %K %Ca %Mg
8 a.m. 2.03 0.24 1.41 0.28 0.20
11 a.m. 1.97 0.25 1.40 0.27 0.19
2 p.m. 1.88 0.23 1.38 0.26 0.18
5 p.m. 1.80 0.22 1.40 0.25 0.18
Signicance ‡ ** ** NS ** **
§ Source: Thein and Gascho (1980).
† TVD = top visible dewlap.
‡ The 1% level of signicance of linear regression is indicated by **. NS = not signicant.
Sufciency Ranges
•
Top Visible Dewlap (approx. 3rd leaf blade lamina)
Macronutrients
N P K Ca Mg S
2.00–2.60% 0.22–0.30% 1.00–1.60% 0.20–0.45% 0.15–0.32%
Micronutrients
Fe Mn Zn Cu B
50–105 ppm 12–100 ppm 16–32 ppm 4–8 ppm 10–50 ppm
Hawaiian Systems
Macronutrients
N P K
Crop logging, leaf blades 3–6, lamina 1.85%
Crop logging, leaf sheaths 3–6 0.08% 2.25%
HSPA, internodes 8–10 0.24–0.35% 0.03–0.04% 0.7–1.0%
DRIS Norms •
DRIS norms have been developed in several areas. The crop has been shown to respond well
to “in crop” corrections made following DRIS analysis. The norms developed in Florida on
muck soils are quite similar to those developed in South Africa on mineral soils (Table 2).

37
Reference Sufciency Ranges — Field Crops
Table 2. Sugarcane TVD leaf blade lamina norms from Florida and South Africa § †
Nutrient Ratio Florida South Africa
N/P 8.706 8.197
N/K 1.526 1.511
K/P 5.633 5.464
Ca/N 0.151 0.128
Ca/P 1.314 1.146
Ca/K 0.222 0.205
Ca/Mg 1.373 1.158
Mn/N 0.113 0.116
Mn/P 0.984 0.962
Mn/K 0.163 0.186
§ Data from Beauls and Sumner (1976) and Elwali and Gascho (1983).
† TVD = top visible dewlap.
Remarks •
The critical values and sufciency ranges presented in this paper are not absolute for all
sugarcane. They are heavily based on the author’s studies on the muck soils in Everglades
Agricultural Area of South Florida and on the top-visible-dewlap leaf blade lamina collected
in the early morning. Muck soils enhance N uptake. As a result, N concentrations in varieties
grown in Florida are generally higher than those produced on mineral soils in other areas.
References •
Beauls ER, Sumner ME. 1976. Application of DRIS approach for calibrating soil and plant
factors in their effects on yield of sugarcane. Proc S Afr Sugar Technol Assoc 50:118–24.
Clements HF. 1980. Crop logging of sugarcane—principles and practices. Honolulu (HI):
University of Hawaii Press.
Elwali AMO, Gascho GJ. 1983. Sugarcane response to P, K, and DRIS corrective treatments
in Florida Histosols. Agron J 75:79–83.
Evans H. 1965. Tissue diagnostic analysis and their interprepation on sugarcane. Proc Int Soc
Sugar Cane Technol 12:156–80.
38
Gascho GJ, Elwali AMO. 1978. Tissue testing of Florida sugarcane. Gainesville (FL):
University of Florida Institute of Food and Agricultural Sciences (IFAS). Belle Glade
Agricultural Research and Education Center Research Report EV-1978-3.
Gascho GJ, Anderson DL, Bowen JE. 1993. Sugarcane. In: Bennett WF, editor. Nutrient
deciencies and toxicities. St Paul (MN): American Phytopathological Society Press.
Srivastava SC. 1992. Sugarcane. In: Wichmann W, editor. IFA world fertilizer use manual.
Paris (France): International Fertilizer Industry Association.
Thein S, Gascho GJ. 1980. Comparison of six tissues for diagnosis of sugarcane mineral
nutrient status. Proc Int Soc Sugar Cane Technol 17:152–62.

39
Reference Sufciency Ranges — Field Crops
Tobacco, Burley C. R. Campbell
Critical Values •
Limited published information:
Magnesium (Mg) 0.2% (whole plant)
Molybdenum (Mo) 0.38% (whole plant), 0.42% (cured leaves)
Sampling Procedures •
The most recent mature or fully expanded leaf (MRML) is the best indicator of nutritional
status. This is the rst leaf back from the growing point that is fully developed. Cell division
is complete, but cell expansion will continue. The MRML is generally the 4th or 5th leaf
back from the bud.
A total of 6 to 10 leaves are required for analysis, depending on size. As leaves become
larger, the lamina from one side of the midrib can be removed from several leaves for a
representative sample. In either case, midribs should be removed before grinding.
Diagnostic samples should be taken at rst signs of a problem. Comparative samples from
“good” and “bad” plants should be taken along with matching soil samples from the root
zones. If symptoms are zonal on the plant, it is helpful to take the MRML sample and a
separate sample of leaves showing the symptoms.
To monitor nutritional status, samples should be taken at lay-by and/or owering.
After topping, the 2nd or 3rd leaf from the top of the stalk is the best indicator sample of
nutritional status.
Samples are shipped to the laboratory in paper containers.

40
Sufciency Ranges •
Most recent mature leaf
Macronutrients (%)
Growth Stage N (%) P (%) K (%) Ca (%) Mg (%) S (%)
Seedling 4.0–6.0 0.2–0.5 3.0–4.0 0.6–1.5 0.2–0.6 0.15–0.6
Early growth 4.0–5.0 0.2–0.5 2.5–3.5 0.75–1.5 0.2–0.6 0.15–0.6
Flowering 3.5–4.5 0.2–0.5 2.5–3.5 0.75–1.5 0.2–0.6 0.15–0.6
Maturity 3.0–4.0 0.2–0.5 2.5–3.5 0.75–1.5 0.2–0.6 0.15–0.6
Micronutrients (ppm)
Growth Stage Fe Mn Zn Cu B Mo
All 50–300 20–250 20–60 5–10 18–75 0.2–1.0
Excessive or Toxic Nutrient Levels
Manganese toxicity can occur at approximately 1000 ppm but is temperature
dependent. Toxicity occurs most often at low temperatures and is generally associated
with low pH.
Important Ratios
The N:S ratio should be less than 18 at all growth stages.
DRIS Norms•
DRIS norms for cured burley have been developed by Evanylo and others (1988).
Remarks•
Sufciency ranges were established based on available references and experience interpreting
analytical results.
References•
Evanylo GK, Sims JL, Grove JH. 1988. Nutrient norms for cured burley tobacco. Agron J
80(4):610–4.
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Miner GS, Tucker MR. 1990. Plant analysis as an aid in fertilizing tobacco. In: Westerman
RL, editor. Soil testing and plant analysis. 3rd ed. Madison (WI): Soil Science Society of
America, Inc [SSSA]. p 645–57. (SSSA book series; 3).

41
Reference Sufciency Ranges — Field Crops
Tobacco, Flue-cured C. R. Campbell
Critical Values •
Limited published information:
Boron (B) 15–16 (bud leaves)
Manganese (Mn) 18–25 (most recent mature leaves)
Magnesium (Mg) 0.2% (most recent mature leaves)
Sampling Procedures •
The most recent mature or fully expanded leaf (MRML) is the best indicator of nutritional
status. This is the rst leaf back from the growing point that is fully developed. Cell division
is complete, but cell expansion continues until maturity. The MRML is generally the 4th or
5th leaf back from the bud.
To evaluate nitrogen status and gain information on ripeness for harvest, samples should be
taken from the upper, middle or lower stalk positions.
Depending on size, a total of 6 to 10 leaves are required for analysis. Laboratory work can
be completed on only one leaf, but it must be representative of the area sampled. As leaves
become larger, lamina from one side of the midrib can be removed from several leaves for a
representative sample. Midribs should always be removed before grinding.
Diagnostic samples should be taken at rst signs of a problem. Comparative samples from
“good” and “bad” plants should be taken along with soil from the root zones.
To monitor nutritional status and ne tune fertilizer programs, samples should be taken at
lay-by and topping. As the plant approaches maturity, samples of lower, middle, and upper
stalk positions can be taken to further evaluate nitrogen status and assess ripeness for harvest.
Samples are shipped to the laboratory in paper containers.

42
Sufciency Ranges•
Macronutrients (%)
Growth Stage Tissue N P K Ca Mg S
Seedling MRML 4.0–6.0 0.2–0.5 3.0–4.0 0.6–1.5 0.2–0.6 0.15–0.6
Early growth MRML 4.0–5.0 0.2–0.5 2.5–3.5 0.75–1.5 0.2–0.6 0.15–0.6
Flowering MRML 3.5–4.5 0.2–0.5 2.5–3.5 0.75–1.5 0.2–0.6 0.15–0.6
Maturity MRML 2.25–3.0 0.17–0.5 1.6–3.0 0.75–1.5 0.2–0.6 0.15–0.6
Harvest Upper leaf 2.0–2.25 0.14–0.3 1.5–2.5 0.75–1.5 0.2–0.6 0.15–0.4
Harvest Middle leaf 1.6–2.0 0.13–0.3 1.5–2.5 1.0–2.0 0.2–0.6 0.15–0.4
Harvest Lower leaf 1.3–1.75 0.12–0.3 1.3–2.5 1.0–2.5 0.18–0.75 0.15–0.4
Micronutrients (ppm)
Growth Stage Tissue Fe Mn Zn Cu B
Seedling MRML 50–300 20–250 20–60 5–10 18–75
Early growth MRML 50–300 20–250 20–60 5–10 18–75
Flowering MRML 50–300 20–250 20–60 5–10 18–75
Maturity MRML 50–300 20–250 20–60 5–10 18–75
Harvest Upper leaf 40–200 20–350 18–60 5–10 18–30
Harvest Middle leaf 40–200 20–350 18–60 4–10 18–30
Harvest Lower leaf 40–200 18–350 18–60 3–10 15–30
Excessive or Toxic Nutrient Levels
Manganese toxicity can occur as concentration approaches 1000 ppm and is usually
associated with low pH.
Important Ratios
The N:S ratio should be less than 18 at all growth stages.
DRIS Norms•
DRIS norms have not been reported for cured ue-cured tobacco.
Remarks•
Sufciency ranges were established based on available references, research, crop monitoring,
and experience interpreting analytical results.
References•
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Miner GS, Tucker MR. 1990. Plant analysis as an aid in fertilizing tobacco. In: Westerman
RL, editor. Soil testing and plant analysis. 3rd ed. Madison (WI): Soil Science Society of
America, Inc [SSSA]. p 645–57. (SSSA book series; 3).
43
Reference Sufciency Ranges
— Forage and Hay Crops —
44

45
Reference Sufciency Ranges — Forages & Hay Crops
Alfalfa C. O. Plank
Critical Values•
None established.
Sampling Procedures•
Sample the top 4 to 6 inches of the plant prior to or at 1/10 bloom stage.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
3.00–5.00% 0.25–0.70% 2.00–3.50% 0.80–3.00% 0.25–1.00% 0.25–0.50%
Micronutrients
Fe Mn Zn Cu B
30–250 ppm 25–100 ppm 20–70 ppm 4–30 ppm 20–80 ppm
Important Ratios
Maintain the N:S ratio between 10:1 and 15:1 for ruminant nutrition.
DRIS Norms•
DRIS norms from populations yielding 3.5 megagrams (metric tons) per hectare per cutting are
given below (Walworth and others 1986). These norms were developed from alfalfa grown in
Georgia using the harvested aerial portion of the plants at approximately 1/10th bloom.
Expression Norms § CV (%) Expression Norms § CV (%)
(N/DM) × 100 2.952 7.2 (Ca/DM) × 100 1.186 13.1
N/P 12.450 19.1 Mg/Ca 0.1365 23.1
N/K 1.499 18.2 Zn/Ca 18.27 24.7
N/Ca 2.534 11.6 Cu/Ca 7.097 19.3
§ Concentrations of N, P, K, Ca and Mg are expressed in dekagrams per kilogram and
those of Zn, Cu and B in milligrams per kilogram.

46
Expression Norms § CV (%) Expression Norms § CV (%)
Mg/N 0.0550 22.6 B/Ca 34.06 20.7
N/Zn 0.1504 24.5 K/Zn 0.1026 27.0
N/Cu 0.4583 26.1 Cu/K 3.431 15.5
B/N 15.09 19.6 B/K 23.13 33.7
(P/DM) × 100 0.2435 15.5 (Mg/DM) × 100 0.1609 19.2
P/K 0.1240 23.8 Zn/Mg 132.60 31.5
P/Ca 0.2163 23.8 Cu/Mg 43.96 28.0
Mg/P 0.6722 21.3 B/Mg 279.50 23.7
Zn/P 90.45 55.6 (Zn/DM) × 106 21.44 49.8
Cu/P 28.68 27.8 Cu/Zn 0.3462 29.0
B/P 185.40 26.2 Zn/B 0.4967 47.4
(K/DM) × 100 2.034 19.1 (Cu/DM) × 106 6.886 26.6
K/Ca 1.938 19.3 B/Cu 7.048 37.7
Mg/K 0.0831 31.9 (B/DM) × 106 44.18 17.9
§ Concentrations of N, P, K, Ca and Mg are expressed in dekagrams per kilogram and
those of Zn, Cu and B in milligrams per kilogram.
Remarks•
DRIS norms developed for some crops may vary somewhat from one geographical region
to another. This is illustrated by the work of Walworth and others (1986) who showed that
norms developed for alfalfa in the Midwest (Erickson and others 1982) differed signicantly
from those developed in Georgia. Soils in the two regions differ appreciably and are believed
to account for the wide differences in Mg and B norms between the regions. Consequently,
when such factors are known, they should be taken into account when selecting both DRIS
norms and sufciency ranges for interpretative purposes.
References•
Erickson T, Kelling KA, Shulte EE. 1982. Predicting alfalfa nutrient needs through DRIS.
Proc 1982 Wisconsin Fert Agric Lime Pest Mgmt Conf 21:233–46.
Kresge CB, Younts SE. 1962. Effect of various rates and frequencies of potassium
application on yield and chemical composition of alfalfa and alfalfa-orchardgrass. Agron J
54:313–6.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Walworth JL, Sumner ME, Isaac RA, Plank CO. 1986. Preliminary DRIS norms for alfalfa in
the southeastern United States and a comparison with midwestern norms. Agron J 78:1046–
52.

47
Reference Sufciency Ranges — Forages & Hay Crops
Coastal Bermuda C. O. Plank and C. R. Campbell
Critical Values•
See remarks.
Sampling Procedures•
Sample the upper half of the plant prior to seed head formation.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
2.00–2.60% 0.20–0.40% 1.50–2.30% 0.25–0.50% 0.10–0.25% 0.15–0.25%
Micronutrients
Fe Mn Zn Cu B
50–200 ppm 20–300 ppm 15–70 ppm 4–20 ppm 5–15 ppm
Important Ratios
N:S = 12 to 16:1 for medium- to high-intensity forage production.
DRIS Norms•
DRIS norms are given in Kelling and Matocha (1990) and Tarpley and others (1985). Tarpley’s
data are as follows.
Nutrient Ratio Mean CV (%) Nutrient Ratio Mean CV (%)
N/P 10.11 11.92 P/Mg 1.48 11.37
N/S 11.85 16.59 S/K 0.12 21.82
N/Ca 7.61 11.60 K/Ca 5.37 17.54
N/Mg 14.94 14.75 K/Mg 10.70 25.10
K/N 0.71 17.74 Ca/Mg 1.97 11.92
P/K 0.14 21.87 S/Ca 0.65 15.02
S/P 0.87 17.57 S/Mg 1.29 18.78
P/Ca 0.76 9.65
48
Remarks •
The lower limit of the sufciency ranges reported above are similar to some reported critical
values (~90% relative yield). Using 90% relative yield as the lower limit of the sufciency
range for Coastal bermuda is most practical for interpreting plant analysis data. This is due
to the rather at slope of the response curves for most fertilizer elements. Thus, setting the
lower limit of the sufciency range at 100% relative yield would not be economically or
environmentally sound. This is particularly true with nitrogen, phosphorus and potassium.
References •
Adams WE, White AW, McCreery RA, Dawson RN. 1967. “Coastal” bermudagrass forage
production and chemical composition as inuenced by potassium source, rate, and frequency
of application. Agron J 59:247–50.
Day JL, Parker MB. 1985. Fertilizer effects on crop removal of P and K in “Coastal”
bermudagrass forage. Agron J 77:110–4.
Eichorn, MM Jr, Nelson BD, Amacher MC, Hallmark WB, Brant MR, Bartkiewicz SA,
Devold L, Fontenot JD. 1987. Effects of fertilizer on potassium on Coastal bermudagrass
grown on Coastal Plain soil. Baton Rouge (LA): Louisiana Agricultural Experiment Station.
Bulletin 782. 73 p.
Kelling KA, Matocha JE. 1990. Plant analysis as an aid in fertilizing forage crops. In:
Westerman RL, editor. Soil testing and plant analysis. 3rd. ed. Madison (WI): Soil Science
Society of America. p 603–43.
Nelson LR, Keisling TC, Rouquette FM Jr. 1983. Potassium rates and sources for “Coastal”
bermudagrass. Soil Sci Soc Am J 47:963–6.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Sedberry JE, Amacher MC, Bligh DP, Curtis OD. 1987. Plant-tissue analysis as a diagnostic
aid in crop production. Baton Rouge (LA): Louisiana Agricultural Experiment Station.
Bulletin 783. 15 p.
Tarpley ML, Robinson DL, Gustavson BK, Eichorn MM Jr. 1985. The DRIS for
interpretation of Coastal bermudagrass analysis. Commun Soil Sci Plant Anal 16:1335–48.
Walker ME, Keisling TC, Marchant WH. 1979. A comparison of solid and liquid fertilizer for
“Coastal” bermudagrass hay production. Soil Sci Soc Am J 43:597–601.
Wilkinson SR, Langdale GW. 1974. Fertility needs of the warm-season grasses. In: Mays
DA, editor. Forage fertilization. Madison (WI): American Society of Agronomy. p 119–45.

49
Reference Sufciency Ranges — Forages & Hay Crops
Tall Fescue G. M. Lessman and W. O. Thom
Critical Values•
N P K
2.5% 0.2% 2.2%
Sampling Procedures •
Samples should be collected every ve to six weeks during growing season before owering.
Collect above ground portion of 20 plants.
Sufciency Ranges •
Actively Growing Plants
Macronutrients
N P K
2.8–3.8% 0.26–0.40% 2.5–3.5%
DRIS Norms •
No DRIS norms have been established.
Remarks •
Forage grasses that contain less than 0.2% Mg are inadequate for grazing and may cause
grass tetany. Fescue containing less than 0.2% Mg will still produce high dry matter yields.
50
References •
Hallock DL, Brown RH, Blaser RE. 1966. Response of Coastal and Midland bermudagrass
and Kentucky 31 fescue to nitrogen in southeastern Virginia. Blacksburg (VA): Virginia
Polytechnic Institute Agricultural Experiment Station. Research Report 112.
Hannaway DB, Bush LP, Leggett JE. 1980. Plant nutrition: magnesium and hypomagnesemia
in animals. Lexington (KY): University of Kentucky Agricultural Experiment Station.
Bulletin 716.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 130 p.
Mayland HF, Grunes DL. 1979. Soil-climate-plant relationships in the etiology of grass
tetany. In: Grass tetany. Madison (WI): American Society of Agronomy. Special Publication
No 35. p 123–75.
Reid RL, Post AJ, Jung GA. 1970. Mineral composition of forage. Morgantown (WV): West
Virginia Agricultural Experiment Station. Bulletin 589T.

51
Reference Sufciency Ranges — Forages & Hay Crops
Orchardgrass and
Smooth Bromegrass S. J. Donohue and H. J. Savoy, Jr.
Critical Values•
None established.
Sampling Procedures •
Samples should be collected ve weeks after cutting (or ve weeks after growth begins in the
spring) and before plants ower. Collect above-ground portion of 20 plants.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
2.50–3.50% 0.25–0.35% 2.50–3.50% 0.30–0.50% 0.15–0.30% 0.20–0.30%
Micronutrients
Fe Mn Zn Cu B
50–250 ppm 50–200 ppm 20–50 ppm 3–10 ppm 5–20 ppm
Important Ratios
Maintain the N:S ratio between 10:1 and 15:1 for ruminant nutrition.
DRIS Norms •
No DRIS norms have been established.
52
References •
Donohoe SJ, Evanylo GK. 1998. Sampling instructions and nutrient sufciency ranges
for tissue analysis. Blacksburg (VA): Virginia Polytechnic Institute and State University.
Publication 452-211.
Donohue SJ, Rhykerd CL, Holt DA, Noller CH. 1973. Inuence of N fertilization and N
carryover on yield and N concentration of Dactylis glomerata L. Agron J 65:671–4.
Gordon CH, Decker AM, Wiseman HG. 1962. Some effects of nitrogen fertilizer, maturity
and light on the composition of orchardgrass. Agron J 54:376–8.
Grifth WK, Teel MR, Parker HE. 1964. Inuence of nitrogen and potassium on the yield
and chemical composition of orchardgrass. Agron J 56:473–45.
Kresge CB, Younts SE. 1963. Response of orchardgrass to potassium and nitrogen
fertilization on a Wickham silt loam. Agron J 55:161–4.
Reid RL, Jung GA, Kinsey CM. 1966. Nitrogen fertilization in relation to the palatability and
nutritive value of orchardgrass. J Animal Sci 25:636–45.
Reid RL, Post AJ, Jung GA. 1970. Mineral composition of forages. Morgantown (WV): West
Virginia Agricultural Experiment Station. Bulletin 589T.
53
Reference Sufciency Ranges
— Vegetable Crops —
54

55
Reference Sufciency Ranges — Vegetable Crops
Bell Pepper E. A. Hanlon and G. J. Hochmuth
Critical Values•
None established.
Sampling Procedures•
The most recent mature leaf should be sampled. Considerable work in bell pepper has shown
that nutrient concentration changes rapidly with stage of growth. For possible correction
of nutrient deciencies, leaves may be sampled just prior to blossoming or at rst blossom
opening. For next season planning of fertilization needs, additional samples from the most
recently mature leaves at early fruit set and early harvest may be useful. Concentrations above
the sufcient range for nutrients that are immobile in the soil are indicative of high soil fertility.
Fertilization with these nutrients in subsequent seasons should be reduced or eliminated.
Sufciency Ranges •
Prior to Blossoming
Macronutrients
N P K Ca Mg S
4.0–5.0 % 0.3–0.5% 5.0–6.0 % 0.9–1.5% 0.35–0.60% 0.3–0.6%
Micronutrients
Fe Mn Zn Cu B
20–150 ppm 30–100 ppm 25–80 ppm 5–10 ppm 20–50 ppm
First Blossom Opening
Macronutrients
N P K Ca Mg S
3.0–5.0% 0.3–0.5% 2.5–5.0% 0.9–1.5% 0.3–0.5% 0.3–0.6%
Micronutrients
Fe Mn Zn Cu B
30–150 ppm 30–100 ppm 25–80 ppm 5–10 ppm 20–50 ppm

56
Early Fruit Set
Macronutrients
N P K Ca Mg S
2.9–4.0% 0.25–0.40% 2.5–4.0 % 1.0–1.5% 0.3–0.4% 0.3–0.4%
Micronutrients
Fe Mn Zn Cu B
30–150 ppm 30–100 ppm 25–80 ppm 5–10 ppm 20–50 ppm
Early Harvest
Macronutrients
N P K Ca Mg S
2.5–3.0% 0.2–0.4% 2.0–3.0 % 1.0–1.5% 0.3–0.4% 0.3–0.4%
Micronutrients
Fe Mn Zn Cu B
30–150 ppm 30–100 ppm 25–80 ppm 5–10 ppm 20–50 ppm
DRIS Norms •
DRIS norms have not been established for bell pepper.
Remarks •
Tabular data agree with the experimental evidence reported in articles listed in the References
section. However, some values are lower than those reported by Jones et. al. (1991). The
differences can be attributed to the fact that these values are based upon measurements within
experiments, compared to mean values observed with time in the analytical laboratory.
References •
Albregts EE. 1971. Effect of nitrogen and potassium on bell pepper grown under paper
mulch. Proc Soil Crop Soc Fla 31:116–8.
Fiskell JGA, Locascio SJ, Singholka S, Martin FG. 1977. Effects of fertilizer N sources, rates
and placement on soil test values for bedded peppers with and without mulch. Proc Soil Crop
Soc Fla 37:183–8.
57
Reference Sufciency Ranges — Vegetable Crops
Hochmuth G, Hanlon E, Hochmuth B. 1992. Response of pepper to N fertilization in a
polyethylene mulch and drip irrigation production system at Live Oak, FL, Spring 1988.
Research Report Suwannee Valley AREC 92-29.
Hochmuth GJ, Hanlon EA. 1995. Commercial vegetable crop nutrient requirements in
Florida. Gainesville (FL): University of Florida Cooperative Extension Service. Special
Publication SP 177.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Publication SS-VEC-42.
Hochmuth GJ, Shuler KD, Gilreath PR, Mitchell RL. 1988. Field testing of Mehlich-I
predicted potassium fertilizer recommendations for mulched pepper. Soil Crop Sci Soc Fla
Proc 47:30–5.
Hochmuth GJ, Shuler KD, Miller RL, Gilreath PR. 1987. Nitrogen crop nutrient requirement
demonstrations for mulched pepper in Florida. Proc Fla State Hort Soc 100:205–9.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 130 p.
Knavel DE, Ellis J, Morrison J. 1977. The effects of tillage systems on the performance and
elemental absorption by selected vegetable crops. J Am Soc Hort Sci 102(3):323–7.
Locascio SJ, Alligood MR. 1992. Nitrogen and potassium source and n-rate for drip-irrigated
pepper. Proc Fla State Hort Soc 105:323–5.
Locascio SJ, Fiskell JGA. 1976. Pepper production as inuenced by mulch, fertilizer
placement, and nitrogen rate. Proc Soil Crop Soc Fla 36:114–7.
Locascio, SJ, Fiskell JGA, Gratez DA. 1985. Nitrogen accumulation by pepper as inuenced
by mulch and time of fertilizer application. J Am Soc Hort Sci 110(3):325–8.
Locascio SJ, Fiskell JGA, Martin FG. 1981. Responses of bell pepper to nitrogen sources. J
Am Soc Hort Sci 106(5):628–32.
Miller CH, McCollum RE, Claimon S. 1979. Relationships between growth of bell peppers
(Capsicum annuum L.) and nutrient accumulation during ontogeny in eld environments. J
Am Soc Hort Sci 104(6):852–7.
Wiedenfeld RP. 1986. Rate, timing, and slow-release nitrogen fertilizers on bell peppers and
muskmelon. HortScience 21(2):233–5.
58

59
Reference Sufciency Ranges — Vegetable Crops
Broccoli E. A. Hanlon and G. J. Hochmuth
Critical Values•
None established.
Sampling Procedures•
The most recently mature leaves should be sampled at heading. Concentrations above the
sufcient range for nutrients that are immobile in the soil are indicative of high soil fertility.
Fertilization with these nutrients in subsequent seasons should be reduced or eliminated.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
3.0–4.5 % 0.3–0.5% 1.5–4.0 % 1.2–2.5% 0.23–0.40% 0.2%
Micronutrients
Fe Mn Zn Cu B
40–300 ppm 25–150 ppm 45–90 ppm 5–10 ppm 30–50 ppm
DRIS Norms •
DRIS norms have not been established for broccoli.
Remarks •
Tabular data agree with the experimental evidence reported in articles listed in the References
section. However, some values are lower than those reported by Jones and others (1991). The
differences can be attributed to the fact that these values are based upon measurements within
experiments, compared to mean values observed with time in the analytical laboratory.
60
References •
Dechak KT, Smith CB. 1990. Yield responses and nutrient uptake of broccoli as affected by
lime type and fertilizer. J Am Soc Hort Sci 115(5):737–40.
Hochmuth GJ, Hanlon EA. 1995. Commercial vegetable crop nutrient requirements in
Florida. Gainesville (FL): University of Florida Cooperative Extension Service. Publication
SP 177.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Publication SS-VEC-42.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Liu L, Shelp BJ. 1993. Broccoli yield and nitrogen composition in response to different
management regimes. Commun Soil Sci Plant Anal 24(1&2):61–84.
Magnico V, Lattanzio V, Sarli G. 1979. Growth and nutrient removal by broccoli. J Am Soc
Hort Sci 104(2):201–3.
Peck NH, MacDonald GE. 1986. Cauliower, broccoli, and brussels sprouts responses
to concentrated superphosphate and potassium chloride fertilization. J Am Soc Hort Sci
111(2):195–201.

61
Reference Sufciency Ranges — Vegetable Crops
Cantaloupe R. M. Lippert
Critical Values•
None established.
Sampling Procedures •
1. When vines are 12 inches long, sample the most recently mature leaves closest to the
growing tip.
2. At ower or initial fruit set, sample the most recently mature leaves closest to the
growing tip. Sample 12–20 leaves, including the petiole.
Sufciency Ranges •
12-inch Vines
Macronutrients
N P K Ca Mg S
4.0–5.0 % 0.4–0.7% 5.0–7.0 % 3.0–5.0% 0.35–0.45% >0.2%
Micronutrients
Fe Mn Zn Cu B
40–100 ppm 20–100 ppm 20–60 ppm 5–10 ppm 20–80 ppm
At Flower Start or Initial Fruit Set
Macronutrients
N P K Ca Mg S
3.0–4.5% 0.25–0.40% 1.8–4.0% 1.8–5.0% 0.3–1.5% >0.2%
Micronutrients
Fe Mn Zn Cu B
30–200 ppm 20–100 ppm 20–60 ppm 5–20 ppm 20–80 ppm
62
DRIS Norms •
None established.
Remarks •
A soil test before planting provides a good assessment of nutrient availability. Since
canteloupes are commonly grown on acidic, sandy soils in the Southeast, a tissue test will
help monitor the availability of leachable nutrients such as nitrogen and sulfur and assess the
level of calcium to avoid blossom-end rot.
References •
Bhella HS, Wilcox GE. 1989. Lime and nitrogen inuence soil acidity, nutritional status,
vegetative growth and yield of muskmelon. J Am Soc Hort Sci 114(4):606–610.
Elamin OM, Wilcox GE. 1986. Effect of magnesium and manganese nutrition on muskmelon
growth and manganese toxicity. J Am Soc Hort Sci 111(4):582–587.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Special Series SS-VEC-42. 62 p.
Locascio SJ. 1993. Cucurbits: cucumber, muskmelon, and watermelon. In: Bennett WF,
editor. Nutrient deciencies and toxicities in crop plants. St. Paul (MN): APS Press. p 123–
130.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Wilcox GE. 1972. Muskmelon response to rates and sources of nitrogen. Agron J 65:694–
697.

63
Reference Sufciency Ranges — Vegetable Crops
Carrot E. A. Hanlon and G. J. Hochmuth
Critical Values•
None established.
Sampling Procedures •
The most recently mature leaf should be sampled about 60 days after planting. For next
season planning of fertilization needs, an additional sample from most recently matured
leaves at harvest may be useful. Concentrations above the sufcient range for nutrients that
are immobile in the soil are indicative of high soil fertility. Fertilization with these nutrients
in subsequent seasons should be reduced or eliminated.
Sufciency Ranges •
60 Days after Seeding
Macronutrients
N P K Ca Mg S
1.8–2.5 % 0.2–0.4% 2.0–4.0 % 2.0–3.5% 0.2–0.5%
Micronutrients
Fe Mn Zn Cu B
30–60 ppm 30–60 ppm 20–60 ppm 4–10 ppm 20–40 ppm
Harvest
Macronutrients
N P K Ca Mg S
1.5–2.5% 0.18–0.40% 1.4–4.0% 1.0–1.5% 0.4–0.5%
Micronutrients
Fe Mn Zn Cu B
20–30 ppm 30–60 ppm 20–60 ppm 4–10 ppm 20–40 ppm
64
DRIS Norms •
DRIS norms have not been established for carrot.
Remarks •
The literature contains few references, but tabular data agree with the experimental evidence
reported in articles listed in the References section. However, some values are lower than
those reported by Jones and others (1991). The differences can be attributed to the fact that
these values are based upon measurements within experiments, compared to mean values
observed with time in the analytical laboratory.
References •
Burdine HW, Hall CB. 1976. Carrot responses to fertilizer levels on everglades organic soils.
Proc Fla State Hort Soc 89:120–5.
Gupta UC, Cutcliffe JA. 1985. Boron nutrition of carrots and table beets grown in a boron
decient soil. Commun Soil Sci Plant Anal 16:509–16.
Hemphill DD Jr, Jackson TL. 1982. Effect of soil acidity and nitrogen on yield and elemental
concentration of bush bean, carrot, and lettuce. J Am Soc Hort Sci 107(5):740–4.
Hipp BW. 1978. Response by carrots to nitrogen and assessment of nitrogen status by plant
analysis. HortScience 13(1):43–4.
Hochmuth GJ, Hanlon EA. 1995. Commercial vegetable crop nutrient requirements in
Florida. Gainesville (FL): University of Florida Cooperative Extension Service. Publication
SP 177.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Publication SS-VEC-42.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Umesh CG, Cutcliffe JA. 1985. Boron nutrition of carrots and table beets grown in a boron
decient soil. Commun Soil Sci Plant Anal 16(5):509–16.

65
Reference Sufciency Ranges — Vegetable Crops
Cauliower E. A. Hanlon and G. J. Hochmuth
Critical Values•
None established.
Sampling Procedures•
The most recently mature leaf should be sampled at buttoning to determine if nutrition
is adequate for the growing season. For next season planning of fertilization needs,
an additional sample from most recently matured leaves at heading may be useful.
Concentrations above the sufciency range for nutrients that are immobile in the soil are
indicative of high soil fertility. Fertilization with these nutrients in the subsequent season
should be reduced or eliminated.
Sufciency Ranges •
Buttoning
Macronutrients
N P K Ca Mg S
3.0–5.0 % 0.4–0.7% 2.0–4.0 % 0.8–2.0% 0.25–0.60% 0.6–1.0%
Micronutrients
Fe Mn Zn Cu B
30–60 ppm 30–80 ppm 30–50 ppm 5–10 ppm 30–50 ppm
Heading
Macronutrients
N P K Ca Mg S
2.2–4.0% 0.3–0.7% 1.50–3.0% 1.0–2.0% 0.25–0.60%
Micronutrients
Fe Mn Zn Cu B
30–60 ppm 50–80 ppm 30–50 ppm 3–5 ppm 30–50 ppm
66
DRIS Norms •
DRIS norms have not been established for cauliower.
Remarks •
The literature contains few references, but tabular data agree with the experimental evidence
reported in articles listed in the References section.
References •
Hochmuth GJ, Hanlon EA. 1995. Commercial vegetable crop nutrient requirements in
Florida. Gainesville (FL): University of Florida Cooperative Extension Service. Publication
SP 177.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Publication SS-VEC-42.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Peck NH, MacDonald GE. 1986. Cauliower, broccoli, and brussels sprouts responses
to concentrated superphosphate and potassium chloride fertilization. J Am Soc Hort Sci
111(2):195–201.
Wall TE, Hochmuth GJ, Hanlon EA. 1988. Calibration of Mehlich-I and -III extractable
potassium for polyethylene-mulched, drip-irrigated cauliower. Soil Crop Sci Soc Fla Proc
48:46–9.
Welch NC, Tyler KB, Ririe D. 1985. Nitrogen rates and nitrapyrin inuence on yields of
brussels sprouts, cabbage, cauliower, and celery. HortScience 20(6):1110–2.

67
Reference Sufciency Ranges — Vegetable Crops
Celery E. A. Hanlon and G. J. Hochmuth
Critical Values•
None established.
Sampling Procedures•
The outer petiole should be sampled about 6 weeks after transplanting. For next season
planning of fertilization needs, an additional sample from the outer petiole at maturity may
be useful. Concentrations above the sufcient range for nutrients that are immobile in the soil
are indicative of high soil fertility. Fertilization with these nutrients in subsequent seasons
should be reduced or eliminated.
Sufciency Ranges •
Six Weeks after Transplanting
Macronutrients
N P K Ca Mg S
1.5–1.7 % 0.3–0.6% 6.0–8.0 % 1.3–2.0% 0.3–0.6%
Micronutrients
Fe Mn Zn Cu B
20–30 ppm 5–10 ppm 20–40 ppm 4–6 ppm 15–25 ppm
Maturity
Macronutrients
N P K Ca Mg S
1.5–1.7% 0.3–0.6% 5.0–7.0% 1.3–2.0% 0.3–0.6%
Micronutrients
Fe Mn Zn Cu B
20–30 ppm 5–10 ppm 20–40 ppm 3–5 ppm 15–25 ppm
DRIS Norms •
DRIS norms have not been established for celery.
68
Remarks•
The tabular data agree with the experimental evidence reported in articles listed in the
References section. However, some values are lower than those reported by Jones and
others (1991). The differences can be attributed to the fact that these values are based upon
measurements within experiments, compared to mean values observed with time in the
analytical laboratory.
References•
[Anonymous]. 1983. Producing celery in the Everglades. American Vegetable Grower 31(2): 39.
Beverly RB. 1987. Celery response to foliar nutritional sprays and acidication of a Histosol.
HortScience 22(6):1271–3.
Beverly RB, Anderson DL. 1988. Response of pot-grown celery to foliar Mn, soil P, and soil
acidication of two Histosols. Soil Crop Sci Soc Fla Proc 47:49–52.
Burdine HW. 1971. The development of pencil stripe in celery: 1B. Nutrient element
composition. Soil Crop Sci Soc Fla Proc 31:37–41.
Burdine HW, Guzman VL. 1963. Some factors associated with the development of pith in
winter grown Everglades celery. Proc Fla State Hort Soc 76:233–8.
Burdine HW, Guzman VL. 1965. The response of some green celery varieties to pH
adjustment with sulfur on Everglades organic soil. Proc Fla State Hort Soc 77:148–56.
Burdine HW, Guzman VL. 1969. Celery cultivar responses to pH adjustment on Everglades
organic soil. J Am Soc Hort Sci 94:520–3.
Burdine HW, Guzman VL. 1969. Nutritional factors affecting nodal cracking of some celery
cultivars. Soil Crop Sci Soc Fla Proc 29:351–62.
Burdine HW, Guzman VL. 1969. Some celery responses to fertilizer levels and soil test
results. Gainesville (FL): University of Florida Everglades Station. Mimeo Report EES 69-
17. 13 p.
Espinoza LA. 1992. Response of celery to phosphorus rate and placement on Histosols [MSc
thesis]. Gainesville (FL): University of Florida. 39 p.
Hochmuth GJ, Hanlon EA. 1995. Commercial vegetable crop nutrient requirements in
Florida. Gainesville (FL): University of Florida Cooperative Extension Service. Special
Publication SP 177.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Sanchez CA, Burdine HW, Guzman VL. 1990. Soil testing and plant analysis as guides for
the fertilization of celery on Histosols. Soil Crop Sci Soc Proc 49:69–72.
Welch NC, Tyler KB, Ririe D. 1985. Nitrogen rates and nitrapyrin inuence on yields of
brussels sprouts, cabbage, cauliower, and celery. HortScience 20(6):1110–2.

69
Reference Sufciency Ranges — Vegetable Crops
Cucumber C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
The most recent mature or fully expanded leaf is the best indicator sample for all growth
stages. This is generally the 4th or 5th leaf from a growing point.
A sample containing 15 to 20 leaves generally represents a uniform eld well.
Problem sampling is done any time during the growing season. Comparative “good” and
“bad” samples help to pinpoint problems.
Samples to monitor nutrient levels are taken at two-week intervals beginning two weeks prior
to bloom and continuing throughout fruiting.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges
Most Recent Mature Leaf — All Growth Stages
Macronutrients
N P K Ca Mg S
4.0–5.0% 0.3–1.0% 3.0–4.0 % 1.2–2.0% 0.25–1.00% 0.20–0.75%
Micronutrients
Fe Mn Zn Cu B
50–300 ppm 25–250 ppm 20–200 ppm 5–60 ppm 25–85 ppm
Important Ratios
The N:S ratio should be less than 18.
The N:K ratio should be 1.2 to 1.8.
70
DRIS Norms•
DRIS norms have not been reported for cucumber.
Remarks•
Sufciency ranges were developed from available references and experience reviewing
analytical results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.

71
Reference Sufciency Ranges — Vegetable Crops
Cucumber, Greenhouse C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
The most recent mature or fully expanded leaf is the best indicator sample for all growth
stages. This is generally the 3rd or 4th leaf from the growing point.
Eight to ten leaves are required for a good sample.
Sampling should commence at the rst sign of a problem, but no less than two weeks before
rst owering for monitoring. Samples should be taken at weekly intervals.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges
Most Recent Mature, or Fully Expanded, Leaf — All Growth Stages
Macronutrients
N P K Ca Mg S
4.5–6.0% 0.3–0.7% 3.5–4.5 % 1.2–1.5% 0.45–0.75% 0.2–0.7%
Micronutrients
Fe Mn Zn Cu B Mo
50–300 ppm 20–300 ppm 20–70 ppm 5–35 ppm 25–85 ppm 0.1–1.0 ppm
Important Ratios
The N:K ratio should be 1.2 to 1.8.
72
DRIS Norms •
DRIS norms have not been reported for greenhouse cucumber.
Remarks•
Sufciency ranges were developed from available references and experience reviewing
analytical results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.

73
Reference Sufciency Ranges — Vegetable Crops
Lettuce, Greenhouse C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
The most recent mature or fully expanded leaf is the best indicator sample for all growth
stages. This is generally the 3rd or 4th leaf from the growing point.
Depending on size, 8 to 10 leaves are adequate for a sample.
Problem samples can be taken at any time during the growing season. Monitoring samples
should be taken at no less than two-week intervals as soon as plants are large enough.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges
Most Recent Mature Leaf — All Growth Stages
Macronutrients
N P K Ca Mg S
4.5–6.5% 0.3–0.8% 6.0–10.0 % 1.0–2.0% 0.35–0.75% 0.2–0.6%
Micronutrients
Fe Mn Zn Cu B Mo
50–200 ppm 20–200 ppm 20–75 ppm 5–15 ppm 25–80 ppm 0.2–1.0 ppm
DRIS Norms •
DRIS norms have not been reported for greenhouse lettuce.
74
Remarks•
Sufciency ranges were developed from available references and experience reviewing
analytical results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.

75
Reference Sufciency Ranges — Vegetable Crops
Muskmelon E. A. Hanlon and G. J. Hochmuth
Critical Values•
None established.
Sampling Procedures•
The most recently matured leaf should be sampled when the vines are about 12 inches long.
Concentrations during harvest can best be judged by sampling the most recently matured leaf
at early fruit set. It is doubtful if addition of fertilizer at or immediately after early fruit set
will inuence crop yield, however. Concentrations above the sufcient range for nutrients
that are immobile in the soil are indicative of high soil fertility. Fertilization with these
nutrients in subsequent seasons should be reduced or eliminated.
Sufciency Ranges •
12-inch Vines
Macronutrients
N P K Ca Mg S
4.0–5.0 % 0.4–0.7% 5.0–7.0 % 3.0–5.0% 0.35–0.45% 0.2+ %
Micronutrients
Fe Mn Zn Cu B
40–100 ppm 20–100 ppm 20–60 ppm 5–10 ppm 20–80 ppm
Early Fruit Set
Macronutrients
N P K Ca Mg S
3.5–4.5% 0.25–0.40% 1.8–4.0% 1.8–4.0% 0.3–0.4% 0.2+ %
Micronutrients
Fe Mn Zn Cu B
40–100 ppm 20–100 ppm 20–50 ppm 5–10 ppm 20–80 ppm
DRIS Norms•
DRIS norms have not been established for muskmelon.
76
Remarks•
Tabular data agree with the experimental evidence reported in articles listed in the References section.
However, some values are lower than those reported by Jones and others (1991). The differences can
be attributed to the fact that these values are based upon measurements within experiments, compared
to mean values observed with time in the analytical laboratory.
References•
Bhella HS, Wilcox GE. 1986. Yield and composition of muskmelon as inuenced by preplant and
trickle applied nitrogen. HortScience 21(1):86–8.
Bhella HS, Wilcox GE. 1989. Lime and nitrogen inuence soil acidity, nutritional status, vegetative
growth and yield of muskmelon. J Am Soc Hort Sci 114(4):606–10.
Brantley BB, Warren GG. 1960. Effect of nitrogen nutrition on owering, fruiting and quality in the
muskmelon. Proc Am Soc Hort Sci 77:424–31.
DeBuchananne DA, Taber HG. 1985. Method of nitrogen application for muskmelons. J Plant Nutr
8(3):265–75.
Elamin OM, Wilcox GE. 1986. Effect of magnesium and manganese nutrition on muskmelon growth
and manganese toxicity. J Am Soc Hort Sci 111(4):582–7.
Elamin OM, Wilcox GE. 1986. Effect of soil acidity and magnesium on muskmelon leaf composition
and fruit yield. J Am Soc Hort Sci 111(5):682–5.
Flocker WJ, Lingle JC, Davis RM, Miller RJ. 1964. Inuence of irrigation and nitrogen fertilization
on yield, quality, and size of cantaloupes. Proc Am Soc Hort Sci 86:424–31.
Gubler WD, Grogan RG, Osterli PP. 1982. Yellows of melons caused by molybdenum deciency in
acid soil. Plant Dis 66(6):449–51.
Hochmuth GJ, Hanlon EA. 1995. Commercial vegetable crop nutrient requirements in Florida.
Gainesville (FL): University of Florida Cooperative Extension Service. Special Publication SP 177.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and interpretations for
vegetable crops in Florida. Gainesville (FL): University of Florida Cooperative Extension Service.
Special Series SS-VEC-42. 62 p.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Lorenz OS, Weir BL, Bishop JC. 1972. Effect of controlled-release nitogen fertilizers on yield and
nitrogen absorption by potatoes, cantaloupes, and tomatoes. J Am Soc Hort Sci 97(3):334–7.
Stark FC, Haut IC. 1958. Mineral nutrient requirements of cantaloupes with reference to nitrogen,
potassium, calcium, magnesium, and boron. College Park (MD): University of Maryland Agricultural
Experiment Station. Bulletin A-93. 33 p.
Tabor HG, Killorn R. 1993. Determination of nitrate in unltered extracts of muskmelon tissue by
ion-selective electrodes. Commun Soil Sci Plant Anal 24(11&12):1231–41.
Wilcox GE. 1972. Muskmelon response to rates and sources of nitrogen. Agron J 65:694–7.

77
Reference Sufciency Ranges — Vegetable Crops
Spinach, Greenhouse C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
The most recent mature or fully expanded leaf is the best indicator sample for all growth
stages. This is generally the 3rd or 4th leaf from the growing point.
Depending on size, 8 to 10 leaves are adequate for a sample.
Problem samples can be taken at any time during the growing season. Monitoring samples
should be taken at two-week intervals as soon as the plants are large enough.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges
Most Recent Mature Leaf — All Growth Stages
Macronutrients
N P K Ca Mg S
4.0–6.0% 0.3–0.5% 3.0–8.0 % 1.0–1.5% 0.4–1.0% 0.2–0.8%
Micronutrients
Fe Mn Zn Cu B Mo
50–200 ppm 25–200 ppm 20–75 ppm 5–15 ppm 25–60 ppm 0.2–1.0 ppm
DRIS Norms •
DRIS norms have not been reported for greenhouse spinach.
78
Remarks•
Sufciency ranges were developed from available references and experience reviewing
analytical results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.

79
Reference Sufciency Ranges — Vegetable Crops
Tomato, Greenhouse C. R. Campbell
Critical Values•
None established.
Sampling Procedures•
The most recent mature or fully expanded leaf is the best indicator sample for all growth
stages. This is generally the 3rd or 4th leaf from the growing point.
Eight to ten leaves are required for a good sample. After drying, the midribs should be
removed and discarded.
Sampling should commence at the rst sign of a problem but no less than two weeks before
owering for monitoring. Samples should be taken at weekly intervals.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges
Most Recent Mature Leaf — All Growth Stages
Macronutrients
N P K Ca Mg S
3.5–5.0% 0.30–0.65% 3.5–4.5 % 1.0–3.0% 0.35–1.0% 0.2–1.0%
Micronutrients
Fe Mn Zn Cu B Mo
50–300 ppm 25–200 ppm 18–80 ppm 5–35 ppm 30–75 ppm 0.1–1.0 ppm
Excessive or Toxic Nutrient Levels
Boron becomes toxic at approximately 200 ppm and can cause distortion and burn of
the growing point. In severe cases, boron tops the plant by injuring the growing point.
In such cases, yield is decreased.
Excess nitrogen is characterized by lengthened internodes and “bullish” growth in the
top of the plant. In severe cases, fruit set is adversely affected. The N:K ratio appears to
be more important than nitrogen concentration in limiting the effects of high nitrogen.
A N:K ratio of 1.2 to 1.8 is desirable.
80
DRIS Norms •
DRIS norms have not been reported for greenhouse tomato.
Remarks•
Sufciency ranges were developed from available references and experience reviewing
analytical results.
References •
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.

81
Reference Sufciency Ranges — Vegetable Crops
Tomato, Trellis C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
The most recent mature or fully expanded leaf is the best indicator sample for all growth
stages. This is generally the 3rd or 4th leaf from the growing point.
A sample containing eight to ten leaves is generally adequate. Midribs are removed after
drying.
Problem sampling is done any time during the growing season. Comparative good and bad
samples help to pinpoint problems.
Sampling to monitor nutrient levels should commence at least two weeks prior to rst bloom
and should continue at two-week intervals throughout the fruiting season.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges
Most Recent Mature Leaf — All Growth Stages
Macronutrients
N P K Ca Mg S
3.5–5.0% 0.3–0.7% 3.0–4.5 % 1.0–2.0% 0.3–0.8% 0.2–0.8%
Micronutrients
Fe Mn Zn Cu B
45–300 ppm 30–300 ppm 18–75 ppm 5–30 ppm 30–75 ppm

82
Excessive or Toxic Nutrient Levels
Boron becomes toxic at approximately 200 ppm and can cause distortion and burn of
the growing point. In severe cases, plants may be topped by the damage followed by
decreased yields.
Excess nitrogen is characterized by lengthened internodes and “bullish” growth in the
top of the plant. In severe cases, fruit set may be affected. The N:K ratio appears to be
more important than nitrogen concentration alone in determining vulnerability to fruit
loss related to excess nitrogen. N:K ratios of 1.2 to 1.8 are ideal.
DRIS Norms •
DRIS norms have not been reported for trellis tomato.
Remarks•
Sufciency ranges were developed from available references and experience reviewing
analytical results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.

83
Reference Sufciency Ranges — Vegetable Crops
Vidalia Onion C. O. Plank
Critical Values•
None established.
Sampling Procedures •
Sample the most recently mature leaves prior to root or bulb enlargement. Avoid dusty
or soil-covered leaves and plants whenever possible. Under normal conditions, rainfall is
frequent enough to keep leaf surfaces fairly free of dust and soil particles. However, when
leaves are dusty, brush or wipe with a damp cloth to remove the contaminants. If this is not
effective or if leaves are covered with spray materials, wash in a mild detergent solution
(0.30%) and rinse in running water. Do not prolong the washing procedures.
Sufciency Ranges
Macronutrients
N P K Ca Mg S
3.10–4.27% 0.26–0.48% 1.98–4.22 % 0.90–1.84% 0.16–0.32% 0.15–0.57%
Micronutrients
Fe Mn Zn Cu B
undetermined 51–149 ppm 16–45 ppm 5–28 ppm 6–15 ppm
Important Ratios
Maintain the N:S ratio between 5:1 and 15:1.
DRIS Norms •
DRIS foliar norms for onions from populations yielding > 45 megagrams (metric tons) per
hectare (n=173) (Caldwell 1991).

84
Expression Mean § CV (%) Expression Mean § CV (%)
N 3.68 16 Mg/K 0.09 69
P 0.37 29 S/K 0.12 47
K 3.10 36 Mn/K 35.75 60
S 0.36 57 Zn/K 10.60 50
Ca 1.37 34 Cu/K 6.62 114
Mg 0.24 33 B/K 3.78 53
Mn 100.14 49 Mg/Ca 0.196 42
Zn 30.21 48 S/Ca 0.31 85
Cu 16.74 70 Mn/Ca 76.86 48
B 10.62 43 Zn/Ca 25.00 70
P/N 1.10 39 Cu/Ca 12.53 62
K/N 0.85 39 B/Ca 8.93 67
Ca/N 0.39 37 S/Mg 1.74 78
Mg/N 0.07 41 Mn/Mg 492.91 79
S/N 0.10 54 Zn/Mg 151.28 72
Mn/N 28.66 53 Cu/Mg 77.18 72
Zn/N 7.82 45 B/Mg 49.56 58
Cu/N 4.83 73 Mn/S 331.70 55
B/N 2.83 40 Zn/S 95.95 52
K/P 9.34 50 Cu/S 59.33 89
Ca/P 4.23 53 B/S 32.00 42
Mg/P 0.69 40
Zn/Mn 0.34 52
S/P 1.03 50 Cu/Mn 0.18 63
Mn/P 312.28 70 B/Mn 0.12 51
Zn/P 88.00 52 Cu/Zn 0.66 83
Cu/P 51.05 86 B/Zn 0.38 41
B/K 30.12 40 B/Cu 0.84 67
Ca/K 0.54 78
§ Concentrations of N, P, K, Ca and Mg are expressed in dekagrams per kilogram and those of Zn, Cu and B in
milligrams per kilogram.
Remarks•
The sufciency ranges were developed from the data of Caldwell (1991) by taking the mean plus
or minus one standard deviation. Onion yields were in excess of 20 tons per acre. The ranges for
N, P, K, Ca and Mg agree very closely with those reported by Pankov (1984) and, with but few
exceptions, are not too dissimilar from those reported by Plank (1989) and Hochmuth and others
(1991). These ranges have been checked against numerous normal- and abnormal-appearing
farmer samples that were analyzed at the University of Georgia Soil Testing and Plant Analysis
Laboratory and are improvements over previously used ranges (Plank 1989).
85
Reference Sufciency Ranges — Vegetable Crops
References •
Caldwell, JO. 1991. Foliar and soil diagnosis and recommendation integrated system (DRIS)
norms for onions (Allium cepa L.) and the effects of N and S on yield and pungency [MSc
thesis]. Athens (GA): University of Georgia.
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Publication SS-VEC-42.
Pankov, VV. 1984. Leaf analysis in relation to onion nutrition. In: Proceedings 6th
international colloquium for the optimization of plant nutrition. Volume 2. Montpellier
(France): AIONP/GERDAT. p 449–56.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
86

87
Reference Sufciency Ranges — Vegetable Crops
Watermelon R. M. Lippert
Critical Values•
None established.
Sampling Procedures •
At ower start or initial fruit set, sample the most recently mature leaves closest to the
growing tip. Sample 12–20 leaves, including the petiole.
Sufciency Ranges
Macronutrients
N P K Ca Mg S
2.5–4.0% 0.25–0.7% 2.25–3.5 % 1.1–2.5% 0.25–0.50% 0.2–0.4%
Micronutrients
Fe Mn Zn Cu B
30–200 ppm 20–200 ppm 20–50 ppm 4–10 ppm 20–40 ppm
DRIS Norms •
DRIS norms have not been established for watermelon.
Remarks •
A soil test before planting provides a good assessment of nutrient availability. Since watermelons
are commonly grown on acidic, sandy soils in the Southeast, a tissue test will help monitor the
availability of leachable nutrients such as nitrogen and sulfur and assess the level of calcium to
avoid blossom-end rot.
88
References •
Hochmuth GJ, Maynard D, Vavrina C, Hanlon EA. 1991. Plant tissue analysis and
interpretations for vegetable crops in Florida. Gainesville (FL): University of Florida
Cooperative Extension Service. Publication SS-VEC-42.
Locascio SJ. 1993. Cucurbits: cucumber, muskmelon, and watermelon. In: Bennett WF,
editor. Nutrient deciencies and toxicities in crop plants. St. Paul (MN): APS Press. p 123–
30.
Locascio SJ, Fiskell JGA. 1966. Copper requirements of watermelons. Am Soc Hort Sci Proc
88:568–75.
Locascio SJ, Fiskell JGA, Lundy HW. 1973. Watermelon response to sulfur-coated urea,
mulches, and nitrogen rates. Fla State Hort Soc Proc 86:201–4.
Locascio SJ, Everett PH, Fiskell JGA. 1968. Effects of phosphorus sources and copper rates
on watermelons. Am Soc Hort Sci Proc 92:583–9.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
89
Reference Sufciency Ranges
— Turf & Lawn Grasses —
90

91
Reference Sufciency Ranges — Turf & Lawn Grasses
Bentgrass C. R. Campbell and C. O. Plank
Critical Values•
None established.
Sampling Procedures •
A representative sample of clippings from a freshly mowed green is the best indicator
of nutritional status. A double handful of clippings is an adequate sample. In as much as
possible, the sample should be taken when clippings are free of foreign matter, including
sand, pine straw, etc.
Samples containing signicant amounts of foreign matter should be processed by the
following procedure.
1. Sand
To remove sand and heavy foreign matter, pour the sample into a 1000-mL beaker
containing distilled water. The bentgrass and lighter materials oat to the surface.
Stir and remove quickly to avoid leaching water-soluble nutrients. Blot dry and
place in dryer.
2. Light-weight foreign matter
After the sample is dry, sieve the sample on a 1-mm screen (No. 18 U.S. Testing
Sieve). The bentgrass falls through the screen while other light-weight particles are
retained for removal.
For problem samples, a matching sample from a “good” green should be taken for
comparison.
Monitoring to ne tune fertility programs and/or maintain records of environmental
stewardship is done by sampling greens monthly. Sampling should follow the same
management sequence monthly to improve usefulness of the data over time.
Samples are loosely packed and shipped to the laboratory in paper containers.

92
Sufciency Ranges •
Clippings from Recently Mowed Green
Macronutrients
N P K Ca Mg S
4.0–5.0% 0.3–0.6% 2.2–3.5% 0.25–0.75% 0.2–0.4% 0.2–0.1%
Micronutrients
Fe Mn Zn Cu B
50–300 ppm 25–300 ppm 20–70 ppm 5–15 ppm 3–20 ppm
Important Ratios
The N:S ratio should be 10 to 15. Ratios over 18 indicate a sulfur deciency. The N:K
ratio should be 1.2 to 2.2.
DRIS Norms •
DRIS norms have not been reported for bentgrass.
Remarks •
Sufciency ranges are based on available literature and experience reviewing analytical
results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.

93
Reference Sufciency Ranges — Turf & Lawn Grasses
Bermudagrass —
‘Tifgreen’ & ‘Tifton-328’ C. R. Campbell and C. O. Plank
Critical Values•
None established.
Sampling Procedures•
Collect representative samples of clippings after routine mowing. A double handful is an
adequate sample size.
Problem-solving samples can be taken at any time there is adequate growth. Comparative
samples from “good” and “bad” areas should be taken to isolate difference between these
areas.
Monitoring samples should be taken monthly to evaluate fertility programs and identify
changes needed to improve growth and quality of sod for the intended purpose.
Samples should be shipped to the laboratory in loosely lled paper containers.
Sufciency Ranges •
Fresh Clippings
Macronutrients
N P K Ca Mg S
3.0–4.0% 0.2–0.4% 1.8–2.25% 0.25–0.5% 0.15–0.3% 0.15–0.65%
Micronutrients
Fe Mn Zn Cu B Mo
50–250 ppm 20–300 ppm 15–70 ppm 5–20 ppm 5–60 ppm 0.1–2.0 ppm
Important Ratios
The N:S ratio should be 10–15 for best growth and quality. Sulfur is decient when
the ratio is greater than or equal to 18.
94
DRIS Norms •
DRIS norms have not been reported for bermudagrass.
Remarks •
Sufciency ranges are based on available literature and experience reviewing analytical
results.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. p 21–8.
95
Reference Sufciency Ranges
— Fruit & Nut Crops —
96

97
Reference Sufciency Ranges — Fruit & Nut Crops
Apple C. O. Plank
Critical Values•
None established.
Sampling Procedures •
Sample 50–100 healthy, mid-terminal leaves on current season’s growth in mid-season (8
to 10 weeks after full bloom).
Sufciency Ranges •
Macronutrients
N § P K Ca Mg S
1.80–2.10% 0.15–0.50% 1.25–1.80% 1.00–2.00% 0.20–0.50% NA
§ These values apply to ‘Golden Delicious’. For all other varieties, the values are
1.90–2.30%.
Micronutrients
Fe Mn Zn Cu B
50–400 ppm 25–200 ppm 20–50 ppm 5–20 ppm 25–60 ppm
DRIS Norms •
DRIS norms have not been established for apple.
Remarks •
Plant analysis is an excellent means of determining the nutritional status and fertilizer needs
of apple. As with many fruit crops, low nutrient levels and/or nutrient imbalances in apple are
often manifested in the fruit before deciency symptoms show on the leaves. Examples are
98
bitter pit due to inadequate Ca and internal corking due to low B. Therefore, it is important
to maintain the nutrient level within the sufciency range to prevent abnormal growth, fruit
color, texture, or shelf life.
In order to make a successful diagnosis, the sample submitted to the laboratory must
represent the overall growing conditions and be properly taken. Always follow the sampling
instructions provided by the laboratory performing the analysis. In addition, there are
several other growth factors that can also inuence the nutrient status of the trees. Apple is
a poor accumulator of Ca, and many producers routinely apply foliar Ca sprays. If the leaf
samples are not properly washed off or if the application of foliar sprays is not noted on
the information sheet accompanying the sample, the analytical results for Ca can be easily
misinterpreted. Therefore, to aid the diagnostician in evaluating the plant analysis data, it
is essential that all available information on cultural and climatic conditions, as well as the
symptomology, be known. Most laboratories provide plant analysis kits containing history
sheets for recording this information.
References •
Hanson E. 1993. Apples and pears. In: Bennett WF, editor. Nutrient deciencies & toxicities
in crop plants. St Paul (MN): American Phytopathological Society Press. p 159–63.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Mulder D. 1950. Magnesium deciency in fruit trees on sandy soils and clay soils in Holland.
Plant Soil 2:145–57.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Shear CB, Faust M. 1980. Nutritional ranges in deciduous tree fruits and nuts. Hort Rev
2:142–64.

99
Reference Sufciency Ranges — Fruit & Nut Crops
Blueberry, Rabbiteye C. O. Plank and M. R. Tucker
Critical Values•
None established.
Sampling Procedures •
Take mature leaves from mid-portion of current season’s growth (lateral shoots, position
4, 5, and 6), during the rst two weeks after harvest.
Sufciency Ranges •
Two Weeks after Harvest
Macronutrients
N P K Ca Mg S
1.20–1.70% 0.08–0.20% 0.35–0.60% 0.25–0.70% 0.14–0.20% 0.11–0.25%
Micronutrients
Fe Mn Zn Cu B
25–70 ppm 25–100 ppm 10–25 ppm 2–10 ppm 12–35 ppm
DRIS Norms •
DRIS norms have not been reported for blueberry.
Remarks •
The sufciency range data given above are a result of a review of the literature, and several
years plant analysis survey data compiled at the University of Georgia Soil Testing and Plant
Analysis Laboratory.
100
References •
Austin ME, Gaines TP. 1984. An observation of nutrient levels in old, unfertilized rabbiteye
blueberry plants. HortScience 19(3):417–8.
Cummings GA. 1986. Personal Communication. N.C. State University, Dept. of Soil Science,
Raleigh, NC.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Spiers JM. 1978. Effects of pH level and nitrogen source on elemental leaf content of
‘Tiftblue’ rabbiteye blueberry. J Am Soc Hort Sci 103(6):705–8.
Spiers JM. 1979. Calcium and nitrogen nutrition of ‘Tiftblue’ rabbiteye blueberry in sand
culture. HortScience 14(4):523–5.
Spiers, JM. 1982. Seasonal variation of leaf nutrient composition in ‘Tiftblue’ rabbiteye
blueberry. J Am Soc Hort Sci 107(2):255–7.
Spiers, JM. 1983. Inuence of N, K, and Na concentration on growth and leaf element
content of ‘Tiftblue’ rabbiteye blueberry. HortScience 18(2): 223–4.

101
Reference Sufciency Ranges — Fruit & Nut Crops
Grape, Muscadine C. O. Plank and C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
Sample the most recent mature leaves adjacent to fruit clusters taken in mid to late
summer, but before nal swelling of the fruit.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
1.65–2.15% 0.12–0.18% 0.80–1.20% 0.70–1.10% 0.15–0.25% 0.15–0.60%
Micronutrients
Fe Mn Zn Cu B
60–120 ppm 60–150 ppm 18–35 ppm 5–10 ppm 15–25 ppm
DRIS Norms •
DRIS norms have not been established for muscadine grape.
Remarks •
The sufciency range data were taken from portions of the data cited in the references and
supplemented with survey data from samples analyzed at the University of Georgia Soil
Testing and Plant Analysis Laboratory.
102
References •
Cummings GA. 1977. Variation in the concentration of certain elements in muscadine grape
leaves related to season, leaf portion and age. J Am Soc Hort Sci 102(3):339–42.
Cummings GA. 1986. Personal communication. N.C. State University, Dept. of Soil Science,
Raleigh, NC.
Cummings GA, Fish AS, Nesbitt WB, Underwood VH. 1973. The inuence of mineral
nutrition and time of year on the elemental concentration of muscadine grapes (Vitis
rotundifolia). Commun Soil Sci Plant Anal 4:211–8.
Cummings GA, Lilly P. 1984. Soil pH rate for fruit and elemental concentration of
muscadine grapes. HortScience 19(6):831–2.
Lott WL. 1952. Magnesium deciency in muscadine grape vines. Proc Am Soc Hort Sci
60:123–31.
Marcy JE, Carroll DE, Cummings GA. 1981. Changes in concentration of certain elements
during maturation of muscadine grapes (Vitis rotundifolia). J Food Sci 46:1891–3, 1897.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. p 43–4.

103
Reference Sufciency Ranges — Fruit & Nut Crops
Peach R. M. Lippert and C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
At mid-season, sample mature leaves from the mid-portion or near the base of the current
season’s terminal growth from at least 50 trees.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
2.75–3.50% 0.12–0.30% 1.30–3.20% 1.50–2.50% 0.25–0.50% 0.12–0.40%
Micronutrients
Fe Mn Zn Cu B
> 60 ppm > 20 ppm 20–50 ppm 5–20 ppm 20–80 ppm
DRIS Norms •
DRIS norms have not been established for peach.
Remarks •
Among the macro and micronutrients, the two required in greatest quantity for good peach
production are nitrogen and potassium. In sandy soils, sulfur may likely be decient. A low
level of calcium or a high level of zinc in the leaves is often an indication of “peach decline.”
Deciencies in manganese, iron, boron, and copper are less prevalent in the Southeast. Leaf
content of iron, manganese, and zinc normally uctuates greatly.
104
References •
Chesness JL, Couvillon G. 1989. Peach tree response to trickle application of water and
nutrients. Athens (GA): University of Georgia Agricultural Station. Research Report 575.
Heckman J. Leaf analysis for fruit trees. Rutgers (NJ): Rutgers University Cooperative
Extension Service. Fact Sheet 627.
Hopnger JA. 1990. Commercial tree fruit production recommendations. Rutgers (NJ):
Rutgers University Cooperative Extension Service and New Jersery Agricultural Experiment
Station.
Johnson RS. 1993. Stone fruit: peaces and nectarines. In Bennett WF, editor. Nutrient
deciencies and toxicities in crop plants. St. Paul (MN): APS Press. p 171–5.
Jones JB, Isaac RA, Skelton BJ. 1976. Nutrient element status of soils and trees for peach
orchards in Georgia and South Carolina. HortScience 11(3):247–8.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.

105
Reference Sufciency Ranges — Fruit & Nut Crops
Pear C. O. Plank and R. M. Lippert
Critical Values•
None established.
Sampling Procedures •
Sample 50–100 healthy, mid-terminal leaves on current season’s growth in mid-season.
Sufciency Ranges •
Macronutrients
N P K Ca Mg S
1.80–2.50% 0.12–0.30% 1.00–2.00% 1.00–2.00% 0.25–0.50% 0.10–0.30%
Micronutrients
Fe Mn Zn Cu B
30–150 ppm 20–200 ppm 20–50 ppm 5–20 ppm 20–60 ppm
DRIS Norms •
DRIS norms have not been established for pear.
Remarks •
Plant analysis is an excellent means of determining the nutritional status and fertilizer needs
of pear. As with many fruit crops, low nutrient levels and/or nutrient imbalances in pear are
often manifested in the fruit before deciency symptoms show on the leaves. Therefore, it
is important to maintain the nutrient level within the sufciency range to prevent abnormal
growth, fruit color, texture, or shelf life.
106
In order to make a successful diagnosis the sample submitted to the laboratory must
represent the overall growing conditions and be properly taken. Always follow the sampling
instructions provided by the laboratory performing the analysis. In addition, there are several
other growth factors that can also inuence the nutrient status of the trees. Therefore, to
aid the diagnostician in evaluating the plant analysis data, it is essential that all available
information on cultural and climatic conditions as well as the symptomology be known.
Most laboratories provide plant analysis kits containing history sheets for recording this
information.
References •
Hanson E. 1993. Apples and pears. In: Bennett WF, editor. Nutrient deciencies & toxicities
in crop plants. St Paul (MN): American Phytopathological Society Press. p 159–63.
Harley CP. 1947. Magnesium deciency in Keiffer pear trees. Proc Am Soc Hort Sci 50:21–
2.
Jones JB Jr, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling,
preparation, analysis, and interpretation guide. Athens (GA): Micro-Macro Publishing. 213 p.
Mulder D. 1950. Magnesium deciency in fruit trees on sandy soils and clay soils in Holland.
Plant Soil 2:145–57.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Shear CB, Faust M. 1980. Nutritional ranges in deciduous tree fruits and nuts. Hort Rev
2:142–64.

107
Reference Sufciency Ranges — Fruit & Nut Crops
Pecan C. O. Plank and C. C. Mitchell
Critical Values•
None established.
Sampling Procedures •
Sample the middle pair of leaets from the mid-portion of terminal growth 56 to 84 days
after catkin fall. The sampling time will vary among states, but in Georgia and Alabama
the preferred sampling time is from July 7 to August 7. Under normal conditions,
rainfall is frequent enough to keep leaf surfaces fairly free from dust and soil particles.
If the leaets are contaminated with residues from foliar sprays, they should be washed
in a mild detergent solution (0.30%) and rinsed in a water bath or running water. Do
not prolong the washing procedure or allow the plant material to “stand” in either the
washing or rinsing solutions.
Sufciency Ranges •
56–84 Days after Catkin Fall
Macronutrients
N P K Ca Mg S
2.50–3.30% § 0.12–0.30% 0.75–2.50% 0.70–1.75% 0.30–0.60% 0.20–0.50%
§ If irrigated, the optimum range is 2.80–3.00%. For the ‘Desirable’ variety, the suf-
ciency range is 2.30–3.00%.
Micronutrients
Fe Mn Zn Cu B
50–300 ppm 100–800 ppm 50–100 ppm 6–30 ppm 15–50 ppm
DRIS Norms •
Preliminary DRIS norms for pecans are given by Beverly and Worley (1992), as follows.

108
Nutrient Ratio Mean CV (%) Nutrient Ratio Mean CV (%)
N § 27.2 9.4 Mn 324 54.5
P 1.4 18.1 Zn 126 60.0
K 10.2 20.9 Cu 9.69 32.4
Ca 14.5 33.4 Mo 6.3 28.1
Mg 3.82 29.9 B 40.1 34.7
Fe 89.4 40.9 Al 380 34.3
N/P 19.8 18.2 Ca/Zn 0.168 75.4
N/K 2.74 20.9 Ca/Cu 1.62 47.8
N/Ca 2.44 130.0 Ca/Mo 2.27 29.2
N/Mg 7.74 36.7 Ca/B 0.325 41.6
N/Fe 0.349 36.4 Ca/Al 0.0109 135.0
N/Mn 1.107 47.4 Mg/Fe 0.0518 45.2
N/Zn 0.306 76.5 Mg/Mn 0.0158 58.3
N/Cu 3.13 41.0 Mg/Zn 0.0438 71.9
N/Mo 4.56 28.0 Mg/Cu 0.470 49.4
N/B 0.746 40.2 Mg/Mo 0.565 42.9
N/Al 0.0257 138.0 Mg/B 0.111 45.0
P/K 0.143 25.4 Mg/Al 0.00389 155.0
P/Ca 0.123 117.0 Fe/Mn 0.354 46.0
P/Mg 0.414 46.5 Fe/Zn 1.04 91.7
P/Fe 0.0175 32.0 Fe/Cu 9.98 42.9
P/Mn 0.00535 41.3
Fe/Mo 18.6 45.8
P/Zn 0.0164 76.4 Fe/B 2.77 46.5
P/Cu 0.163 38.9 Fe/Al 0.0958 149.0
P/Mo 0.301 25.6 Mn/Zn 3.23 83.6
P/B 0.0412 40.0 Mn/Cu 35.4 56.6
P/Al 0.00143 154.0 Mn/Mo 70.0 45.3
K/Ca 0.948 151.0 Mn/B 10.2 65.0
K/Mg 2.97 46.3 Mn/Al 0.328 171.0
K/Fe 0.134 35.9 Zn/Cu 14.8 57.6
K/Mn 0.0409 50.7 Zn/Mo 21.5 54.7
K/Zn 0.121 77.3 Zn/B 3.69 66.5
K/Cu 1.23 41.7 Zn/Al 0.125 183.0
K/Mo 1.86 32.4 Cu/Mo 1.70 41.1
K/B 0.319 53.6 Cu/B 0.276 43.8
K/Al 0.0104 137.0 Cu/Al 1.00954 148.0
Ca/Mg 4.13 42.0 Mo/B 0.183 71.5
Ca/Fe 0.193 54.2 Mo/Al 0.00586 159.0
Ca/Mn 0.0583 58.5 B/Al 0.0384 170.0
§ N, P, K, Ca and Mg expressed in g/kg (parts per thousand); other elements in mg/kg (ppm).
109
Reference Sufciency Ranges — Fruit & Nut Crops
Remarks •
The sufciency ranges given above were taken from Plank (1989). The ranges have been
developed over the past 25–30 years utilizing research data from various sources, surveys,
and plant analysis summaries.
References •
Beverly RB, Worley RE. 1992. Preliminary DRIS diagnostic norms for pecan. HortScience
27(3):271.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.
Sparks D. 1976. Magnesium nutrition and the pecan—a review. Pecan South 3(3):384–7.
Sparks D. 1977. Methods of predicting the nutrient needs of nut trees. [place unknown]:
Northern Nut Growers Association. 68th Annual Report. p 25–30.
Sparks D. 1977. Nitrogen—re-evaluation of its effects on pecan yield and nut growth. Pecan
South 4(May/June):16–9.
Sparks D. 1978. Nutrient concentrations of pecan leaves with deciency symptoms and
normal growth. HortScience 13(3):256–7.
Sparks D. 1978. Predicting the nutrient needs of pecan—a review. Pecan South 5(6):280–4.
Sparks D. 1993. Threshold leaf levels of zinc that inuence nut yield and vegetative growth
in pecan. HortScience 28(11):1100–2.
Worley RE. 1974. Effect of N, P, K and lime on yield, nut quality, tree growth, and leaf
analysis of pecan (Carya illinoensis W.). J Am Soc Hort Sci 99:49–57.
Worley RE. 1985. Use of leaf analysis for basing N application for Stuart pecans. Proc SE
Pecan Growers Assoc 78:79–83.
110

111
Reference Sufciency Ranges — Fruit & Nut Crops
Strawberry —
Annual Hill Culture C. R. Campbell and G. S. Miner
Critical Values•
None established.
Sampling Procedures•
The most recent mature trifoliate and petiole are the best indicator samples.
Concentrations of essential elements are determined on the trifoliate. Nitrate nitrogen is
determined on the petioles.
Fifteen trifoliates and petioles are required for a representative sample.
Samples are collected during fall growth as needed to solve problems and monitor crop
development. Intensive biweekly sampling is initiated when spring growth begins and
continued throughout owering and harvest (approximately March 1–May 30) in North
Carolina. Petioles are removed from trifoliates at the sampling site.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges •
Most Recent Mature Trifoliate — All Growth Stages
Macronutrients
N P K Ca Mg S
3.0–4.0% 0.2–0.4% 1.1–2.5% 0.5–1.5% 0.25–0.45% 0.15–0.40%
Micronutrients
Fe Mn Zn Cu B
50–300 ppm 30–300 ppm 15–60 ppm 3–15 ppm 25–50 ppm
Excessive or Toxic Nutrient Levels
Boron becomes toxic as concentrations approach 200 ppm B. Excess boron results in a
marginal leaf burn beginning rst on lower leaves and progressing up the plant. Severe
cases result in >10% yield loss.

112
Petioles from Most Recent Mature Trifoliate — Spring Growth
DRIS Norms•
DRIS norms have not been reported for strawberry.
Remarks •
Sufciency ranges were adopted from California studies and modied for North Carolina
conditions based on numerous eld studies.
Petiole nitrate nitrogen values well above the sufcient zone during fruiting result in soft
fruit.
References•
Hockmuth G, Albregts E. 1994. Fertilization of strawberries in Florida. Gainesville (FL):
University of Florida Cooperative Extension Service. Circular 1141. 4 p.
Miner GS, Poling EB, Carroll DE, Nelson LA, Campbell CR. 1997. Inuence of fall
nitrogen and spring nitrogen-potassium applications on yield and fruit quality of ‘Chandler’
strawberry. J Am Soc Hort Sci 122(2):290–5.
113
Reference Sufciency Ranges
— Ornamentals & Flowers —
114

115
Reference Sufciency Ranges — Ornamentals & Flowers
Ornamental Cabbage C. R. Campbell
Critical Values•
None established.
Sampling Procedures •
The most recent mature leaf is the best indicator.
Ten to 15 leaves are required for a representative sample.
Samples are collected during vegetative growth as soon as plants are large enough.
Samples are shipped to the laboratory in paper containers.
Sufciency Ranges •
Vegetative Growth — Most Recent Mature Leaf
Macronutrients
N P K Ca Mg S
3.5–4.5% 0.2–0.6% 3.0–4.0% 0.5–1.0% 0.2–0.4% 0.2–1.0%
Micronutrients
Fe Mn Zn Cu B Mo
50–300 ppm 20–250 ppm 20–75 ppm 3–10 ppm 20–40 ppm 0.1–2.0 ppm
Important Ratios
The N:S ratio should be between 10 and 15. Ratios above 18 are considered high and
indicate a need for sulfur.
116
DRIS Norms •
DRIS norms for ornamental cabbage have not been reported.
Remarks •
Sufciency ranges were developed based on experience and published ranges for similar
crops.
References •
Mills HA, Jones JB Jr. 1996. Plant analysis handbook II: a practical sampling, preparation,
analysis, and interpretation guide. Athens (GA): MacroMicro Publishing, Inc.
Plank CO. 1989. Plant analysis handbook for Georgia. Athens (GA): University of Georgia
Cooperative Extension Service. 64 p.

117
Reference Sufciency Ranges — Ornamentals & Flowers
Poinsettia C. R. Campbell
Critical Values•
Levels at which deciency symptoms are evident and growth and development are affected.
N P K Ca Mg S Fe Mn Cu B Mo
3.50% 0.15% 1.00% 0.50% 0.20% 0.05% 30 ppm 15 ppm 1 ppm 15 ppm 0.5 ppm
Sampling Procedures •
The most recent fully expanded or mature leaf is the best indicator of nutritional status.
This is the rst fully expanded leaf below the growing point.
Sampling is initiated as soon as plants are large enough that leaf removal will not limit
further development.
Sampling is discontinued when bracts near full development.
Depending on size, approximately 10–15 leaves are required per sample.
Sufciency Ranges •
All Growth Stages
Macronutrients
N P K Ca Mg S
4.0–6.0% 0.3–0.6% 1.5–3.5% 1.00–1.75% 0.3–1.0% 0.1–0.3%
Micronutrients
Fe Mn Zn Cu B Mo
50–300 ppm 20–250 ppm 20–60 ppm 2–10 ppm 25–75 ppm 1–5 ppm

118
Important Ratios
The N:S ratio should not exceed 18.
Excessive or Toxic Nutrient Levels
N P K Cl F Li Mn B
7.3% 0.9% 4.0% 3.0% 5 ppm 20 ppm 1000 ppm 200 ppm
Boron toxicity is common where irrigation water contains 0.5 ppm B or higher.
Excess boron causes a marginal leaf burn that begins on older leaves. Toxicity
symptoms progress up the plant with time. Leaf margins contain very high
concentrations of boron.
Lithium toxicity is associated with some water supplies and vermiculite deposits
containing high concentrations of this element. Symptoms include marginal burn on
older leaves. Leaf margins contain very high concentrations of lithium.
DRIS Norms •
DRIS norms for poinsettia have not been reported.
Remarks •
Critical values were taken from the work of Ecke and others (1990) and modied based on
experience. Sufciency ranges and toxicity values were taken from work of Ecke and others
(1990) and modied based on experience.
References •
Ecke P Jr, Matkin OA, Hartley DE. 1990. The poinsettia manual. Encinitas (CA): Paul Ecke
Poinsettias. p 121.
119
Reference Sufciency Ranges
— Tree Crops —
120

121
Reference Sufciency Ranges — Tree Crops
Fraser Fir C. R. Campbell and L. E. Hinesley
Critical Values•
None reported.
Sampling Procedures •
Needles from most recent mature foliage in the upper half of the tree are the best
indicator sample. Do not sample needles from the leader or top whorl.
For monitoring, the preferred sampling time is in the fall after dormancy.
Comparative samples from “good” and “bad” trees can be taken for diagnosing problems
at any growth stage. The best indicator sample is needles from the current year’s growth.
Samples should contain 15–20 laterals from ten or more trees representing the eld.
Sufciency Ranges •
Current Year’s Growth after Dormancy
Macronutrients
N P K Ca Mg S
1.5–2.0% 0.2–0.6% 0.6–0.8% 0.45–0.60% 0.10–0.20% 0.08–0.20%
Micronutrients
Fe Mn Zn Cu B
40–300 ppm 30–300 ppm 18–75 ppm 5–10 ppm 18–30 ppm
Important Ratios
The Fe:Mn ratio should be greater than or equal to 1.

122
DRIS Norms•
DRIS norms have been published by Beverly (1991), Hockman and others (1989), and Kopp
and Burger (1990).
Expression Mean CV (%) Expression Mean CV (%)
N 2.28% 3.53 Mg/N 0.04 10.92
P 0.23% 11.61 P/K 0.26 11.62
K 0.88% 6.45 Ca/P 1.71 20.06
Ca 0.38% 16.59 Mg/P 0.43 12.62
Mg 0.10% 10.59 Ca/K 0.44 18.19
P/N 0.10 14.47 Mg/K 0.11 12.00
N/K 2.61 7.23 Mg/Ca 0.25 10.70
Ca/N 0.17 15.40
Remarks•
Sufciency ranges are based on available research and modied based on experience
interpreting plant analysis results.
DRIS norms are based on work of Beverly (1991), Hockman and others (1989), and Kopp
and Burger (1990).
Nutrient concentrations vary with maturity of foliage. With the exception of copper,
concentrations of most elements increase between mid-summer and late fall.
References•
Hinesley LE, Campbell CR. 1991. Crooked leaders and nutrition in Fraser r Christmas trees.
Can J Forest Res 22:513–20.
Hinsley LE, Wright RD. 1989. Biomass and nutrient accumulation in Fraser r Christmas
trees. HortScience 24(2):280–2.
Hockman JN, Burger JA, Smith DW. 1989a. A DRIS application to Fraser r Christmas trees.
Hockman JN, Burger JA, Smith DW. 1989b. Special and temporal variability of foliar
nutrient levels in fraser r christmas trees. Forest Sci 35(2):632–9.
Rathfon RA, Burger JA. 1991. Diagnosis and recommendation integrated system (DRIS)
nutrient norms for Fraser r Christmas trees. Forest Sci 37(4):998–1010.
Robarge WP, Pye JM, Bruck RJ. 1989. Foliar elemental composition of spruce-r in the
southern Blue Ridge province. Plant and Soil 114:19–34.
Warren SL, Campbell CR, Skroch WA. 1990. Nutrient concentrations and their seasonal
patterns in Fraser r and Norway spruce grown in seven vegetation management programs. J
Am Soc Hort Sci 115(1):62–7.
