
Precision and
Bias
of
Estimates
of
Larval Mortality
Nancy
C.
H.
Lo,
John
R.
Hunter,
and
Roger
P.
Hewitt
ABSTRACT The results of four ichthyoplankton
surveys conducted during January through April
1984
off
the coast of California were used
as
the
basis for Monte Carlo simulation of populations of
northern anchovy,
Engrnulia
mordax,
larvae. The
simulated populations were sampled and
larval
mortality rate was calculated, using established
analytical procedures. Results may
be
used
to deter-
mine the precision of an estimate of
larval
mortal-
ity rate and to determine the number of plankton
tows required to detect
a
difference in mortality
rates between two surveys. The estimated mortality
rate was found to
be
biased high when the larval
growth rate is overestimated and biased low when
the growth rate is underestimated. The bias is
asymmetrically distributed and greatest when the
assumed growth substantially overestimates the
real growth. The results justify interannual com-
parisons of larval anchovy mortality rates when
interannual variation in
larval
growth is less than
twofold. The results
also
indicate that the sample
size required for adequate precision of estimates of
mortality rates is modest compared to that required
for adequate representation of the spawning season
and
larval
habitat.
The early life
stages
of several fish have been
extensively studied as they are the link between
the present adult stock and some future recruit-
ment to the adult stock. Frustrated with the
apparent lack of a clear relationship between
stock and recruitment, fishery scientists have
focused attention on events during the larval
stage and their ultimate effect on survival to the
juvenile and adult stages. Several hypotheses
have been proposed (e.g., Hjort
1913);
however,
an understanding of the precision and accuracy
of estimates of larval mortality
rates
is neces-
sary
to distinguish among them (Gulland
1971).
This
paper draws upon
our
experience with the
northern anchovy,
Engraulis
mordax,
to ad-
dress this issue.
We focus on three questions:
1)
What is the
Nancy
C.
H.
Lo,
John
R.
Hunter,
and
Roger
P.
Hewitt
Southwest Fisheries Center, National Marine Fisheries
Service,
NOAA,
P.O.
Box
271,
La
Jolla,
CA
92038.
116.nseript
accepted
hh
1969.
Fishery
Bulletin.
U.S.
87:
399-416.
minimum number of plankton tows required to
estimate the mortality rate of young larvae
(<20
days old) for a given coefficient of variation?
2)
What is the minimum number of plankton tows
required to detect a difference in the mortality
rates of young larvae between two surveys?
3)
How does violation of the assumption
of
a con-
stant growth model affect the estimate of larval
mortality?
Several biases associated with sampling north-
ern anchovy larvae have been identified and
quantified. Pelagic ichthyoplankton are caught
by lowering a fine-mesh net to a depth below the
larval habitat and by steadily retrieving it to the
surface of the ocean (Smith and Richardson
1977).
Variability in the volume of water filtered
per unit of depth affects the number
of
larvae
captured; Ahlstrom
(1948)
formulated the “stan-
dard haul factor” to adjust for this bias. Larvae
are
extruded through the meshes of the
sam-
pling gear: retention rates can be expressed as a
function of larval length and mesh size (Lenan
1972;
Zweifel and Smith
1981;
Lo
1983).
Larvae
also evade capture
as
evidenced by differences in
the night and day catch
rates
(Ahlstrom
1954;
Smith
1981):
retention rates can be expressed
as
a
function of larval length and the diurnal time of
capture (Hewitt and Methot
1982).
The apparent
length of larvae is affected by abrasion from the
sampling net and by the preservative solution:
live larval length may be expressed
as
a function
of preserved larval length and the duration of
the plankton tow (Theilacker
1980).
The application of these corrections yields
unbiased estimates of the density of larvae in
each of several length categories. Age-specific
variations
in
growth introduce variability in the
duration of time that
a
larva of given length is
vulnerable to capture. The density of larvae
divided by the duration of growth through each
length category yields estimates of the number
of larvae
of
a given age produced per unit sea-
surface-area per unit time, which is termed
larval production (Hewitt and Methot
1982).
Yolk-sac larvae growth has been described
as
a
function of temperature (Zweifel and Lasker
399

FISHERY
BULLETIN:
VOL.
8i.
NO.
3.
1989
tality rates were subsequently calculated assum-
ing a set of growth rates (i.e., no interannual
variation). By comparing the calculated mortal-
ity rates to a known rate, the magnitude of
biases may be investigated.
1976;
Lo
1983). Growth of feeding larvae has
been described as a function of season (Methot
and Hewitt 19801). Interannual variations in
growth have not been described, and in the ab-
sence of additional information, a larval growth
model with constant coefficients is used for all
years. The set of coefficients encompassed tem-
perature
effects as well as seasonal effects. The
rate of decline of larval production with age
represents the mortality rate (Hewitt 1981).
In actual practice, a negative binomial-
weighted model (Bissel1972) has been employed
to convert length-specific distributions of larval
density to unbiased age-specific distributions of
larval production, assuming one
set
of size-spe-
cific extrusion and voidance rates (Zweifel and
Smith 1981; Hewitt 1982; Hewitt and Methot
1982; Hewitt and Brewer 1983; Picquelle and
Hewitt 1983, 1984;
Lo
1985). The negative bi-
nomial distribution is recommended for describ-
ing
sample counts of fish
eggs
and larvae (Smith
and Richardson 1977); the distribution is capable
of adequately describing patchy spatial distribu-
tion patterns. The arithmetic means of these dis-
tributions describe the mortality (or production)
of larvae with age.
Although the negative binomial-weighted
model produces an estimate of the variance of
the mean density
at
a particular age, each age-
specific distribution is unique because of the
spatial dispersal of the larvae (Hewitt 1981). The
variance of the mean density is underestimated
as
the extrusion and avoidance are assumed to
be
constant, and the variance about the mortal-
ity curve (hence, the variance of the mortality
rate)
is not easily determined. In the simulation,
random variation of avoidance of the net and
extrusion through the meshes of the net were
included
so
that the variance of the mortality
rate
might
best
be evaluated. The approach used
here is to construct a simulated population, sam-
ple it with simulated surveys, and estimate the
mortality rate of larvae, using the procedures
described above. By conducting many surveys,
the accuracy and precision of the estimates of
mortality
rates
may be investigated'.
Potential biases in estimating larval mortality,
introduced by assuming no interannual variation
in growth, were our main concern and were in-
vestigated by simulation. Growth rates were
varied when constructing the populations; mor-
~
'Methot,
R.
D., Jr..
and
R.
P.
Hewitt.
1980.
A generalized
growth curve
for
young
anchovy
larvae:
derivation and
tabular
ex-
ample. SWFC Admin. Rep. LJ-80-17,
8
p.
400
METHODS
A
Monte Carlo simulation model (Fig.
1)
was
employed to address the questions pertaining to
the biases and precision of the estimate of larval
mortality.
A
population of anchovy larvae was
constructed using observed seasonal and geo-
graphic distributions.
A
known mortality rate
was imposed on the population and sampling ef-
fort was varied over time and space. Known
sampling biases were imposed and then adjusted
for using the same techniques for calculating
larval mortality rate
as
have been used on real
surveys. Several hundred simulated surveys
were conducted to
assess
the accuracy and pre-
cision of the estimates of mortality
rates.
Sim-
ulated larval growth was also varied to deter-
mine the sensitivity of the estimates of mortality
rates
to an assumption of constant larval growth.
The details of this simulation are outlined in the
following paragraphs.
Larval
Population
A
series of CalCOFI' ichthyoplankton cruises
conducted in 1984 (Fig.
2)
was used as a basis for
constructing the population of larvae in the
ocean. The total abundance of anchovy larvae at
each station was adjusted for extrusion of small
larvae through the meshes of the net (Fig.
3)
and
avoidance of the net by large larvae (Fig.
4).
The
adjusted catches were then stratified by geo-
graphic region (Fig. 2), month, and tempera-
ture.
The negative binomial distribution was
fit
to the observations (positive tows only) in each
region-month-temperature cell owing to the
patchiness
of
larvae and the fact
that
the mean
larval abundance is less than the standard devia-
tion in general (Table
1).
Samples were ran-
domly drawn from these distributions (where
the variate was the total number of
larvae
<9.25
mm
per
station) to conduct a simulated survey.
'California Cooperative Oceanic Fisheries Investigations
(CPICOFI)
is
a
co~ortium
of
marine institutions engaged
in
long-
term
monitoring
and
atudy
of
the peke
ecology
of
the
California
Current. Lnrge-de iehthyoplnnkton surveys have
been
conducted
Since
1949.
See
Hewitt
1988,
Reid
1988,
and Smith
and
Moser
1988
for
reviews.

""CtlO" "lth
para.
FI(;I.RE
1.-Flow
chart
of
the
simulation,
Allocation
of
Sampling
Effort
Simulated population encountered by plankton
tows
was computed according to their distribu-
tion in 1984 by month and region (Table
1).
The
portion of simulated tow that contained an-
chovy larvae was similarly determined (Table
2:
App.). In this way, the sample size (number of
tows) could be varied and yet still retain the
spatial and temporal distribution of sampling ef-
fort that was used in 1984. The time of the sim-
ulated tows was assigned by randomly selecting
a value from a Gamma distribution fitted to the
actual time between tows in each region (Table
3;
APP.).
Larval Mortality Rate
Because it was found that anchovy larvae suf-
fer higher mortality during the first-feeding pe-
riod than during later
stages,
a Pareto function
describes the survival of anchovy larvae younger
than
20
days adequately (Hewitt and Brewer
1983;
Lo
1985, 1986). In the present study, we
used the Pareto function to assign
age
to the
larvae in the population (Table
1;
Fig.
5;
App.).
401

FISHERY
BVLLETIS:
VOL.
87.
N(I
.!.
1Hh!l
123
FIGURE
2.-Description of the seasonal and geographic distribution of sampling effort on a series
of
ichthyoplankton cruises
conducted
off
the coast of California in
1984.
The abundance of anchovy larvae at each station is indicated by the height
of
the
“tree.” Stations are grouped into geographic regions
4
through
14.
402

I
1.0
R
R
’
1
l+exp(4.36-
1.66Lc)
I
0‘
FIGIVIE
3.-Retention
of
anchovy larvae not extruded through the meshes
of
a plankton net
constructed
of
0.506
mm
nylon
(Lo
1983).
R
is
the portion
of
larvae.
of
preserved length
L,.
retained in the net.
-c
-
1-
01
I
I
0
12
24
Hour
FIGURE
4.-Retention
of
anchovy larvae
which have not avoided capture (Heaitt and
Methot
1982).
R
is the portion
of
larvae.
of
preserved length
L,.
retained in the net.
DN,
is the length-specific dayhight catch
ratio.
where:
L,
2.5
3.75
4.75
5.75
6.75
7.75
8.75
DNL
1.67
1.47
1.46
I
.27
1.21
1.16
1.13
403

FISHERY
BI'LLETIN:
VOL.
Si.
NO.
3.
I%Y
TAELE
1
-Simulated population of anchovy larvae based on a series
of
ichthyoplankton
surveys conducted in
1984
Tabulated values are the parameters
(rn
and k)
of
negative
binomial distributions'
fit
to
the DoDulation stratified by month. reaion. and ternoerature
Temperature
'C
513" 13 1"-14' 14 1"-15" ;15'
Month Region
rn
k
rn
k m k
rn
k
1 4
5
7
8
9
11
13
14
2 4
5
7
8
9
11
13
14
3 4
5
7
8
9
11
13
14
4
5
7
8
9
11
13
31.25
20.25
270.50
2.00
34.00
56.80
6.80
0.25
558.1 7
0.25
270.50
2.00
34.00
56.80
6.80
0.25
2.00
0.25
4.00
1 17.50
0.00
14.50
6.80
0.25
2.00
0.25
4.00
11 7.50
0.00
14.50
6.80
0.39
0.62
0.60
0.55
0.37
0.64
0.62
0.60
0.55
0.37
0.40
5.39
0.50
0.00
2.14
0.37
0.40
5.39
0.50
0.00
2.14
0.37
x
x
x
X
x
x
x
x
x
63.00
0.25
270.50
2.00
34.00
56.80
6.80
0.25
120.50
0.25
270.50
2.00
34.00
56.80
6.80
0.25
2.00
0.25
7.33
117.50
0.00
14.50
6.80
0.25
2.00
0.25
7.33
11 7.50
0.00
14.50
6.80
0.34
0.62
0.31
0.60
0.55
0.37
0.32
0.62
0.31
0.60
0.55
0.37
0.40
5.39
0.50
0.00
2.14
0.37
0.40
5.39
0.50
0.00
2.14
0.37
X
X
X
I
x
x
X
27.50 0.51
0.25
x
147.08 0.25
44.00
0.22
136.00 0.41
56.80
0.55
6.80 0.37
0.25
x
22.50 0.97
0.25
x
147.08 0.25
44.00 0.22
136.00 0.41
56.8G
0.55
6.80
0.37
0.25
x
2.00
0.40
0.25
522.33 1.07
520.00 0.20
150.50 0.78
147.00 1.22
6.80
0.37
0.25
x
2.00
0.40
0.25
x
522.33
1.07
520.00
0.20
150.50
0.78
147.00
1.22
6.80 0.37
27.50 0.51
0.25
x
619.10 0.14
98.60 0.22
51.00 4.33
56.80
0.55
6.80 0.37
0.25
22.50 0.97
0.25
x
619.10 0.14
98.60 0.22
51.00
4.33
56.80
0.55
6.80 0.37
0.25
x
2.00
0.40
0.25
790.60 1.38
206.30 0.45
150.50 0.78
514.30 0.45
6.80 0.37
0.25
x
2.00 0.40
0.25
x
790.60 1.38
206.30 0.45
150.50 0.78
514.30 0.45
6.80 0.37
14 0.25
x
0.25
x
0.25 0.25
x
'Negative binomial distnbution where
(xi
k-
111
[m/m
+
k)]'*
[k/m
+
k)]'ior
x
=
0.1.2.3..
p'x=
*
=
X!(k
-
1)l
'Poisson distribution was
used
where
RX
=
x)
=
(m'e-m)/xl
tor
x
=
0.1.2.3,
A
two-step Gompertz growth curve (Fig.
6)
was
used to determine the corresponding larval
length. The length at age was generated based
on a normal distribution with mean equal to the
length computed from the Gompertz growth
curve and a standard deviation equal to
0.2
times
the length. (The standard deviation is normally
proportional to the mean length at age.) The
coefficient of variation of
0.2
was arbitrarily
404
chosen because no direct estimate of the stan-
dard deviation was available. These simulated
larvae, with assigned ages and lengths, com-
posed the catches.
Sampling
Biases
The simulated catches were reduced to ac-
count for the effects of extrusion and avoidance.

TABLE 2.-Distribution of sampling effort during January through April 1984 by
region and month, where
p(/)
is
the
propoflion
of
tows
for
monfh
iand
Xp(r)
=
1,
is the proportion
of
tows made in region ]during month iand
IqCjiij
=
1, and
r(ili)
is the proportion
of
positive tows for region jduring month
I
and
0
5
f(j/i)
L
1.
The number
of
tows is indicated by
N,
and the positive tows are indicated by
n
(i.e.. those tows which contained anchovy larvae)
January February March April Total
i=
1 2
3
4
N=
139
89 67
54
349
n=
55
55
47 19 176
P(
d
0.40
0.26 0.19 0.15
(0.50)
Region
q
r
q
r
q
r
q
r
4 0.14 0.43
0.28 0.29
0
-
0.36 0.14
5
0.14 0.06 0.20
0.06
0
-
0.15 0
7 0.25
0.90
0.21
1.00
0.26 0.94 0.26 0.93
8
0.04
0.67
0.01 1.00
0.14 0.88 0.02 1.00
9 0.130.06
0.160.63 0.021.00
0.160.11
11 0.14
0.33
0.06
0
0.24 0.88 0.05
0
13 0.09
0.27 0.02
1.00 0.25
0.41
0
-
14 0.07
0.08
0.06
0.17 0.09
0 0
-
1
.oo
1
.oo
1
.oo
1
.oo
TABLE 3 -Two parameters describing
Gamma distributions' fit to the time be-
tween tows minus the constant in each
region Each
of
these distributions is
shined by the addition
of
the constant
listed The constant
is
the minimum time
(hours) between two positive tows
I
I
I
I'
th
t
Ays
1
FIGURE 5,-Pareto model
of
larval production where larval mor-
tality
is
assumed
to
decline with increasing age
(Lo
1985
and
1986).
P,
is
the daily production
of
larvae at age
t;
p
is
the mortal-
ity coefficient: and th is the age at hatch.
Regional
a
p
Constant
4 0.275 43.71 2
5 0.510 3.92 4
7 0.291 34.93 2
8 0.346 42.19 3
9 0.838 39.47 4
11 0.714 5.03 2
13 0.561 21.08 3
14
0.500
69.00 4
'Gamma
distribution where
X>O
409

FISHERY
BrLLETIK
VOL
h7
KO
d,
19XV
L-4.25
fort56.28days
fort >6.28 days
4.1
e--rn’
L-27
(27)
Where:
aT=
aT
exp
(bT
x
TEMPERATURE)
=
0.11
exp
(0.12
x
TEMPERATURE)
am
=
(am-
b,
x
MONTH)-’
-
(22.48-0.83xMONTH)-’
FIGURE
6.-Temperature-dependent
and season-dependent larval
growth curves (Methot and Hewitt 1980;
Lo
1983). Compertz models
are used
to
describe each
growth
phase where
aT
is the temperature-
dependent growth coefficient and
a,
is the season-dependent grouZh
coefficient.
The fraction,
p,
of larvae extruded through the
mesh or avoiding the net was generated by a
sample mean of a binomial random variable,
g,
with parameters
N
and
P.
The parameter:
N
was set to
50
and
P
was the length-specific ex-
trusion rate or avoidance rate from the same
equations used to construct the population from
the 1984 surveys. Thus
p
equaled
y/50.
Although
p
has a mean of
P,
it was not necessarily equal to
P
for each simulation run. The live lengths of
larvae were reduced to account for the effects of
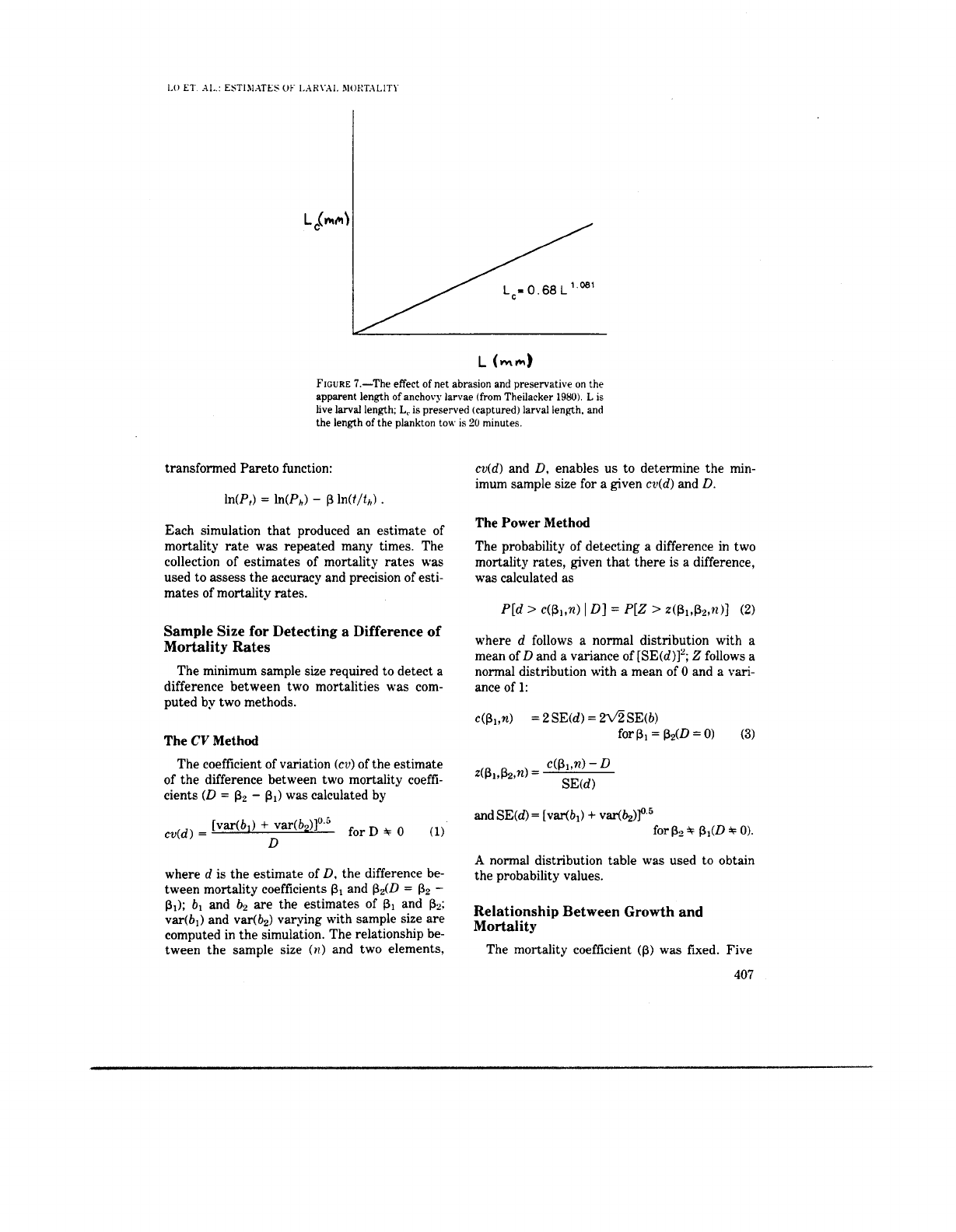
net abrasion and preservaton effects (Theilacker
1980;
Fig.
7).
A standard haul factor was select-
ed from the observed normal distribution of this
variate (mean
=
4.96,
SD
=
0.567)
and used to
index the volume of water filtered per unit of
depth sampled. These catches then formed the
raw material for the mortality estimation pro-
cedure.
406
Estimating Mortality Rate
The larvae in each catch were grouped into
1
mm length categories. A weighted negative bi-
nomial distribution was fitted to each length
category where the original variate was the
number of larvae (of a given length category) per
station. Using this procedure, each observation
was weighted for the effects
of
sampling biases
(extrusion, avoidance, volume of water filtered,
growth and shrinkage). The final variate was the
number of larvae (of a given age) produced per
day per
0.05
m2 of sea surface. The rate at which
larval production declines with time was defined
as
the mortality rate. For the Pareto model, the
mortality rate
was
assumed to decline with age
and mortality was indexed by the mortality coef-
ficient
(PI.
For the simulations described in this
report,
p
was estimated
as
the slope of the log-
L
(mm)
FIGURE
7.-The effect
of
net abrasion and preservative on the
apparent length
of
anchovy larvae
(from
Theilacker
1980).
L
is
live
larval
length;
L,
is preserved (captured) larval length. and
the length
of
the plankton tow. is
20
minutes.
transformed Pareto function:
In(Pt)
=
In(Ph)
-
P
In(l/th)
.
Each simulation that produced an estimate of
mortality rate was repeated many times. The
collection of estimates of mortality rates was
used to assess the accuracy and precision of esti-
mates
of
mortality rates.
Sample Size for Detecting a Difference
of
Mortality Rates
The minimum sample size required to detect a
difference between two mortalities was com-
puted by two methods.
The
CV
Method
The coefficient of variation
(cv)
of the estimate
of
the difference between two mortality coeffi-
cients
(D
=
P2
-
PI)
was calculated by
where
d
is the estimate of
D,
the difference be-
tween mortality coefficients
PI
and
P2(D
=
p2
-
PI);
bl
and
b2
are the estimates of and
P2;
var(bl) and var(b2) varying with sample size are
computed in the simulation. The relationship be-
tween the sample size
(71)
and two elements,
cv(d)
and
D,
enables
us
to determine the min-
imum sample size for a given
cv(d)
and
D.
The Power Method
The probability of detecting a difference in two
mortality rates, given that there is a difference,
was calculated
as
P[d
>
c(p1,n)
I
D]
=
P[Z
>
z(PI,~~,?z)]
(2)
where
d
follows a normal distribution with a
mean of
D
and a variance of
[SE(d)]';
Z
follows a
normal distribution with a mean of
0
and a van-
ance of
1:
c(Pl,n)
=2SE(d)=2fiSE(b)
for
PI
=
P2(D
=
0)
(3)
A
normal distribution table was used to obtain
the probability values.
Relationship Between
Growth
and
Mortality
The mortality coefficient
(P)
was fixed. Five
407

FISHERY
BULLETIN.
VOL.
87.
NO
3.
198Y
From Figure
8
and the above expression,
cr
may
be expected to be
0.10,0.06,
or
0.05
for
20,60,
or
100 positive tows.
For
1)
>
100,
may be ex-
pected to decrease at a slow rate. Thus a survey
of
120
tows, yielding
60
positive tows, is suffi-
cient to estimate the mortality coefficient with
an expected
CY
=
0.06.
Data from annual surveys
conducted between 1980 and 1987, where the
portion of positive tows ranged from 0.47 to 0.98,
are also shown on Figure
8.
The variation of
b,
as
related to sample size during 198047, follows
the relationship estimated from a single year’s
data and implies that the relationship can be
used as a guide for sample size determination.
populations were constructed with data from a
single region-month stratum using five combina-
tions of growth coefficients for yolk-sac
(a~,
a
temperature-specific coefficient) and feeding lar-
vae
(a,,,,
a season-specific coefficient) (see Table
8).
Each population was sampled repeatedly and
an average mortality coefficient calculated as-
suming standard growth coefficients. These
mortality coefficients were then compared with
the fixed mortality coefficient used to construct
the populations.
RESULTS
The simulation model was used to estimate the
following:
1)
the mortality coefficients and their
standard errors for various sample sizes when
the true mortality coefficient was fixed,
2)
the
difference between two mortality coefficients
and its standard error for various sample sues,
and
3)
the mortality coefficients, assuming var-
ious growth rates.
Estimates
of
p
with Various Sample Sizes
The mortality Coefficient
(p)
wa5 fixed at
1.5
for the inshore area (regions 4,
7,
8,
11, and 13;
Fig. 2) and at
0.05
for the offshore area (regions
5,
9,
and 14). The lower coefficient was required
to generate simulated catch curves similar to
those observed in offshore areas. The low mor-
tality coefficient observed in offshore areas was
likely the result of transport of older larvae from
inshore to offshore regions (Power 1986). The
average mortality coefficient
(p),
weighted by
area of each region, was 1.41.
For each sample size
(50,
100,
200,
300, and
400
plankton tows)
100
computer runs were
made, and an estimate of the mortality coeffi-
cient
(b)
was calculated. The mean mortality
coefficient, its standard error, and the coefficient
of variation
(cv)
are listed in Table 4 for each
sample size. The mean mortality coefficient for
all sample sizes, except
50,
slightly overesti-
mated the true value of
p
=
1.41. The
CV
de-
creased with increasing sample size.
The relationship between cv and the number
of positive tows
(n)
was quantified by assuming
that half of the tows contained anchovy larvae
(the actual portion of positive tows in 1984 was
0.5)
(Table 2).The curve (Fig.
8)
may be de-
scribed by the power function:
cv(b)
=
0.418
n-0.47
TAELE
4.--Mean. standard error
(SE).
and coef-
ficient of variation (cvj
of
estimates of the mor-
tality coefficient
(b)
for various sample sizes
(N),
with
50%
positive for anchovy larvae
(n
=
0.5
N),
from
100
computer
runs
of
each simulated
Survey.
N
n
mean
SE
cv= SE/mean
50
25
1.39
0.13 0.09
100
50
1.43
0.09
0.06
200
100
1.44
0.06
0.04
300
150
1.44
0.06
0.05
400
200
1.43
0.05
0.03
Estimates
of
D
with Various Sample Sizes
The mortality coefficient
((3)
was fixed at 1.0,
1.5,2.0,2.5, and 3.0 for the inshore area (regions
4, 7, 8, 11, and 13). The inshore area was rela-
tively well sampled and contained relatively high
abundances of larvae; the proportion of positive
stations in these regions was approximately 0.6
(Tables
1,
2). Estimated mortality coefficients
(b)
were determined for five simulated popula-
tions (corresponding to each of the five mortality
coefficients
(p))
using sample sizes of
50,
100,
and
200
plankton tows with 608 of them positive
for anchovy larvae.
The average estimated mortality coefficient
and its standard error were determined after 100
computer runs and listed in Table
5.
As
ex-
pected, standard errors decreased with in-
creased sample size. The estimated mortality
coefficient was biased slightly low for
p
<
2
and
biased slightly high for
p
>
2.
The biases are
negligible although they appeared to increase in
magnitude
as
p
departed from
2.
The estimates
of mortality
rates
and their standard errors were
used to determine minimum sample size by two
methods.
408

LO
ET.
AL
ESTIMATES
OF
1.4RYAL
hlORTA1.ITY
0.15
I
0.14
-
0.13
-
0.12
-
0.11
-
0.1
-
0.09
-
0.08
-
0.07
-
0.08
-
0.05
-
0.04
-
0.03
-
0.02
cv
(b)
0
1984
I
01
I I I
1980
0
1981
1982
.I
POSITIVE
TOWS
(n)
FIGURE
&-The relationship between the coefficient
of
variation, cv(b). and the number
of
positive tows, n. derived
from
the results
of
the simulation.
1980-87
survey results are
also
plotted.
The
CV
Method
The
CZI
of the estimate of the difference be-
tween two mortality coefficients, cv(d) (Equa-
tion
(l)),
was calculated for various mortality
differences and sample sizes using the data listed
in Table
5.
The cv(d) decreases linearly with the
difference between mortality coefficients
(D),
increases linearly with the absolute value of the
larger of the two mortality coefficients
(Pe).
and
exponentially declines with increasing sample
size
(N,
n)
(Table
6).
The required sample size
was thus estimated by regressing the number of
TABLE 5.--Mean and standard error
(SE)
of estimated mor-
tality coefficient based on
100
computer runs. Five popula-
tions were simulated, each with a different mortality coeffi-
cient
(0).
Simulated surveys used three sample sizes
(N)
with
60%
of the plankton tows positive for anchovy larvae
tnl.
Sample size
Mn)
P
5WO)
lOO(60)
Zoo(
120)
mean
SE
mean
SE
mean
SE
1.00
0.90
0.100 0.91
0.075 0.93 0.058
1.50
1.44 0.090
1.44
0.064
1.44
0.060
2.00 1.98
0.120 1.99
0.087 2.01 0.058
2.50 2.56
0.110 2.57
0.097 2.58 0.065
3.00 3.18
0.170 3.18
0.100 3.18 0.075
positive tows on
p2,
D,
and ln[cv(d)]:
n
=
-101
+
24.8
p2
-
150
D
-
128
ln[cw(d)]
.
For example, estimating the difference be-
tween two estimated mortality coefficients,
when the true mortality coefficients are
3.0
and
TABLE 6.4oefficient
of
variation of the esti-
mate
of
the difference between two mortality
coefficients.
cv(d).
calculated for various mortal-
ity differences,
D.
and sample sizes,
n.
The
number of positive tows,
n,
was
60%
of the total
number
of
tows.
Sample size
Mn)
P2
-
P1
50(30) lOO(60) 200(120)
0
=
0.5
1.5
-
1.0' 0.268
0.196 0.166
2.0
-
1.5 0.300
0.210
0.166
2.5
-
2.0
0.320
0.260 0.170
3.0
-
2.5 0.400
0.270 0.190
2.0
-
1.0 0.156
0.115
0.082
2.5
-
1.5 0.142
0.116 0.088
3.0
-
2.0
0.208
0.133
.
0.095
2.5
-
1.0 0.100
0.082
0.058
3.0
-
1.5 0.130
0.079
0.064
D=
1.0
D=
1.5
409

FISHERY
BCLLETIK
VOL
87.
KO
3.
19x9
mortality coefficient
(p)
using the data listed in
Table 5:
SE(b)
=
0.356
12-u
469
2398
2.0
(pl
=
2.0,
p2
=
3.0,
D
=
l.O),
with a
cv(d)
=
0.15, will require
=
67
positive tows from each
population. With
70
positive tows from each pop-
ulation, approximately
95%
of the sample differ-
ences can be expected to be between
0.70
and
1.30 (1.0
f
2
*
0.15).
The Power Method
The standard error of the estimated mortality
coefficient,
SE(b),
was
modeled
as
a function of
the number of positive tows,
~l,
and the true
The probabilities of detecting a difference be-
tween two mortality coefficients, given that
there is a difference (this
is
referred to as the
power of the'test), were calculated for various
sample sizes and listed in Table
7.
The power
increases
as
the difference of mortality coeffi-
cients increases, and it
is
equal to the level of
TABLE 7.-Probability of detecting a difference between two mortality coeffi-
dents. given one of the mortality coefficients
(p,).
the
true
difference
(D
=
p2
-
PI).
and the number of positive
tows
(n).
Because of symmetry about
D
=
0.
partial figures are listed.
True difference
IDI
n
-2.0
-1.5
-1.0
-0.5
0.5
1.0
1.5
2.0
p1
=
1.0
10
20
30
40
p,
=
1.5
10
20
30
40
50
60
p,
=
2.0
10
20
30
40
50
60
$1
=
2.5
10
20
30
40
50
60
70
pi
3.0
10
20
30
40
50
60
70
80
90
0.62 0.96
1.00
1.00
0.86 1.00 1.00 1.00
0.96 1.00 1.00 1.00
1.00 1.00
1.00 1.00
0.50
0.50
0.97
0.79 0.75 1.00
0.93
0.90
1.00
0.98 0.96 1.00
1.00 0.99 1.00
1.00
1.00 1.00
0.96
0.40
0.43
0.92
1.00 0.70 0.70 1.00
1.00 0.86
0.86 1.00
1.00 0.93 0.92 1.00
1.00 0.98 0.96 1.00
1.00 1.00
1.00 1.00
1.00 0.91
0.34
0.48
1.00 0.99
0.59
0.58
1.00 1.00
0.76 0.75
1.00 1.00 0.86 0.86
1.00
1.00 0.93
0.91
1.00 1.00 0.96 0.95
1.00 1.00 1.00 1.00
1.00 0.99
0.82 0.27
1.00 1.00
0.98
0.50
1.00 1.00 1.00 0.66
1.00
1.00 1.00
0.78
1.00 1.00
1.00 0.86
1.00 1.00
1.00 0.91
1.00
1.00 1.00
0.95
1.00 1.00 1.00 0.97
.oo
.oo
.00
.00
.OO
.00
1.00 1.00 1.00 1.00
__
410

1.0
ET
AL
:
k:STI!d.4TES
OF
1AKV.IL
!dI)RT.II,ITY
significance
(a
=
0.05)
when the difference is
zero. The power is symmetrical about
D
=
0;
thus, only partial figures were given in Table
7.
For example, if the true difference was
0.5
and
one of the mortality coefficients was 2.0, with a
probability of 0.86, a sample size of
30
positive
tows from each of two populations will detect a
significant difference in their mortality coeffi-
cients. The probability would be only 0.76 if one
of the mortality coefficients was 2.5. In general,
to achieve the same probability of detecting a
given difference between mortality coefficients,
a larger sample size
is
required for a larger
p.
To
detect a significant difference with a probability
of
0.96,
when the true difference is 0.5 and one of
the mortality coefficients is 1.0,
30
positive tows
are required from each population. If
p
=
2.5,
however, 60 positive tows are required to detect
the same difference with a probability of
0.95.
If
the difference is greater than 1, at most
20
posi-
tive tows from each population would be suffi-
cient.
The two methods serve different purposes.
The
CP
method provides a
95%
confidence inter-
val for the difference. The Power Method as-
signs a probability to the detection of a differ-
ence, but provides no information on the magni-
tude of the difference.
Estimates
of
p
with Various Growth
Rates
Mortality is defined as the decline of produc-
tion with larval age. Thus an overestimate of
larval age, predicted from an underestimate of
growth rate, will underestimate mortality rate.
Similarly, an overestimate of growth rate will
result in an overestimate of mortality rate.
The mortality coefficient
(p)
was fixed at 1.5.
Data from February, region
7,
temperature
15T,
were used to construct five populations,
corresponding to five combinations of growth
coefficients for yolk-sac and feeding larvae
(Table
8).
Each population was surveyed
50
times with a sample size of 50 plankton tows.
The estimated mortality Coefficient
(b)
was cal-
culated by assuming standard growth coeffi-
cients for February, region
7,
temperature 15°C
(Table
8).
When the population growth coeffi-
cients
(CX,,~)
were underestimated by the stan-
dard coefficients, the estimated mortality coeffi-
cient
(b)
was less than
p
=
1.5; conversely when
growth
was
overestimated, the mortality coeffi-
cient was also overestimated.
Because the yolk-sac stage is short, relative to
the feeding stage, we can reasonably assume
that the growth coefficient for feeding larvae
(a,,,)
has the largest effect on the estimated mor-
tality coefficient
(b).
When the estimated mortal-
ity coefficient is plotted against
a,,,
(Fig.
9).
it is
apparent that the bias in estimating mortality
rate, caused by errors in the assumed growth
rate,
is
asymmetrical: greater when actual
growth is slower than assumed growth and
smaller when actual growth is faster than as-
sumed. When the actual growth was half the
assumed rate, the mortality coefficient was over-
estimated by 804; when the actual growth was
double the assumed rate, the mortality coeffi-
cient was underestimated by only 16% (Table
8).
The coefficient,
a,,,,
determines the instan-
taneous growth rate (IGR) at age
t
as the IGR
=
a,,,
In(L,/Lo) exp[-a,,,(t
-
to)]
where L,
is the maximum fish length, and Lo is the min-
imum fish length for
t
>
6.28 days (Fig. 6).
Large value of
a,,,
implies that the IGR is large
for the small value
of
age
t,
and the IGR de-
creases rapidly as the fish ages. Because both
the IGR and the instantaneous mortality rate
(IMR
=
p/t)
are two different nonlinear func-
tions of age
(t),
the relationship between these
two coefficients
(an,
and
p)
is also nonlinear and
thus the bias
is
asymmetric.
TABLE
8
-Five sets
of
coefficients
for
two-step Gompertz
growth curves (Fig
6)
used to simulate five populations
Also
listed are the standard coefficients used in the analy-
sis
of
survey data for region
7
in February with a ternpera-
ture of
15°C
The estimated mortality coefficient
(b)
is
listed as average
of
50
computer runs The true mortality
coefficient
(4)
was
1.5
ar
bT
aT
am
bm
am
b
0.11
0.06 0.27
44.96
0.83 0.023 2.70
0.11
0.24
4.05
11.24
0.83 0.104 1.26
0.22
0.12
1.33 22.48 0.42
0.046 1.41
0.11
0.09
0.42 33.72
0.83
0.031 1.90
0.22
0.12
1.33 16.86 0.83
0.066 1.31
Standard
coefficients:
0.11 0.12 0.67 22.48 0.83 0.048
DISCUSSION
AND
CONCLUSIONS
The simulation model and
its
methodology have
general applicability to larval fish of many
species, although these results apply directly to
estimates of northern anchovy larval mortality
rates derived from CalCOFI surveys. Results
may differ because of differences in the param-
411

FISHERY
UL'LLETIN
VOL
87.
KO
3
lY8Y
3-
2-
1-
b
assumed
growth
am
-048
I
I
1
0
0.05
0.1
0.15
FIGURE
9.-The estimated mortality coefficient
(b)
is biased by errors in the assumed
poWh
coefficient for feeding larvae,
a,,,
=
0.48.
The true mortality coefficient
(P)
was
1.5.
eter values and their variances. Nevertheless,
most ichthyoplankton sampling problems are
sufficiently similar
so
that the results derived for
anchovy provide a general idea of the sample size
required for adequate precision of larval mortal-
ity estimates. (When using the regressions
derived in this study to estimate sample size,
parameter values should be within the range
used in the simulation
[1.0
5
p
5
3.0,
0.5
5
D
5
2.01.
Values outside these ranges could lead to
unreliable estimates of sample size.) The results
also provide an assessment of the effect of biased
growth rates on estimates of larval mortality
rate, which has general applicability to many
species.
significant overestimate of mortality.
Another problem arises from the choice of
study areas. Many specimens must be collected
over a short period to assess growth, starvation,
and other condition factors. If sites are selected
that contain larval densities that are high, rela-
tive to the average density for the entire habitat,
and patchy, the effect will be to increase the
variance, because the variance is often positively
correlated with the density
of
larvae (Smith and
Richardson
19771,
and thereby reduce the power
to detect differences in mortality rate between
sites. The simulations were based on large
regions of anchovy habitat and therefore under-
estimate sample size required to detect mortal-
ity rate differences between small areas of high
larval abundance.
Caveats
Application to Site-Intensive Studies
Application to Other Species
Small-scale site-intensive studies may be con-
ducted to study underlying mechanisms of larval
mortality rate by measuring larval condition,
growth, starvation rates, and mortality rate in
small segments of the habitat. Such studies have
greater problems with bias and precision than
the CalCOFI surveys where the entire spawning
habitat
is
sampled.
As
noted above, an import-
ant potential bias is the transport of larvae in or
out of the study area. Taggart and Leggett
(1987)
noted that failure to account for advective
losses of larvae from a small bay resulted in a
412
A key difference between larval anchovy and
most other species of larval fishes is that an-
chovy are very abundant. The simulation results
indicate that surprisingly few positive tows are
needed to detect relatively small differences in
mortality rates. In the regions considered in the
simulation,
50-608
of the tows were positive,
and the number of larvae caught
per
tow aver-
aged
125,
with
88%
<
10
mm in length. For
a
less
abundant species, the proportion of positive
tows and the average number caught per tow
would be much lower, and many more tows

LO ET
AL
ESTlBlATES
OF
L.ARV.41.
hlORTALITI’
would be required to attain the same level
of
precision.
Application
to
CalCOFI
Surveys
Three key assumptions underlie the use
of
the
CalCOFI time series of larval mortality esti-
mates for hypothesis testing:
1)
a stable age
distribution prevails (i.e., abundance
of
several
cohorts of larvae at one moment in time is repre-
sentative of one cohort
as
it ages through time),
2)
variations in observed mortality rate repre-
sent true natural variations and not sampling
error, and
3)
use of the same larval growth par-
ameters for all years does not bias the estimates
of mortality rate.
The
first
assumption was not addressed in this
study. It implies negligible immigration and
emigration of larvae and continuous production
of spawn. The CalCOFI surveys are designed to
encompass the anchovy spawning habitat and
thus minimize inaccuracies caused by transport
of larvae in and out of the survey area. For a
species with a broad temporal spawning curve
and with repeated spawning by individuals
(9-16%
of the females spawn each night; table
7,
Fiedler et al.
1986),
unbiased estimates of mor-
tality rate may be obtained by pooling plankton
tows conducted throughout the spawning season
(table
6,
Hewitt and Methot
1982).
With smaller
surveys and shorter time periods, the assump-
tion
of
a stable age distribution may not be suit-
able, and estimates of mortality rates may be
biased.
With regard to the second assumption, our
simulations indicate that the time series of daily
mortality rate of anchovy larvae represents pre-
dominantly real differences owing to biological
variation rather than random variation. Recent
CalCOFI ichthyoplankton surveys (Table
9)
yielded between
36
and
236
positive tows per
spawning season. The simulation model indicates
that sample sizes
>80
are sufficient to detect a
difference of
0.5
or more in the mortality coeff-
cient
(p)
between years (Table
7).
When all pos-
sible pairs for the eight surveys
(1980-87)
are
compared,
12
of the
28
comparisons had a differ-
ence
>0.5
(Table
9).
Results
of
our simulation
imply that the precision
of
past surveys was ade-
quate, and the interannual variation in mortality
rate
(p
ranged from
1.22
in
1980
to
2.14
in
1986)
is real.
Because larval mortality rate
is
age-depen-
dent (IMR
=
p/t)
with high mortality occurring
during the onset of feeding and decreasing there-
after, variations in daily mortality rates can be
typified by “large differences concentrated in a
short period
of
time” and thus be easily detect-
able (Gulland
1971).
The critical issue in compar-
ing mortality rates does not appear to be one
of
precision but rather one
of
obtaining a represen-
tative sample.
With regard to the third assumption, the
simulation also indicated that the risk of intro-
ducing a large bias in estimates
of
mortality
rates by using a single family of standard growth
curves is relatively
low.
A
large bias would be
expected only when the standard growth curves
overestimated the actual growth by a factor
of
two or more. It is unknown how frequently the
standard growth curve generates this large bias,
for lack of data on variability of larval growth
rates from year to year in the field.
TABLE
9.-Nurnber
of
tows
positive for anchovy larvae
(n) and mortality coeffi-
cients
(p)
for CalCOFl
ichthyoplankton surveys
conducted during January
through April 1980-87.
P
Year n
1980
197 1.22
1981
236 1.53
1982
69 1.81
1983
65
2.05
1984
176 1.47
1985
37 2.03
1986
83 2.14
1987
36 1.98
CONCLUSIONS
These simulations validate the use
of
CalCOFI
survey information to
test
hypotheses regarding
larval survival and recruitment (Butler
1987,
Peterman et al.
1988).
The sample size required
for adequate precision of estimates of mortality
rates is modest relative to the one required for
adequate representation of the spawning season
and habitat of a major marine stock such as the
northern anchovy.
As
stated, the critical issue in
comparing mortality rates does not appear to be
precision of the estimates but rather how well
the sample represents the population.
ACKNOWLEDGMENTS
We would like to acknowledge Paul Smith,
who outlined the main consideration necessary
413

FISHERY
BL'L1,ETlii
VOL.
87,
NO.
3,
1989
with anchovy egg and larval abundance: temperature
dependent incubation time, yolk-sac powth rate and
egg and larval retention
in
mesh nets. NOAA Tech.
Memo. NMFS-SWFC-31.
32
p.
1985.
Egg production of the central stock of northern
anchovy
1951-83.
1986.
Modeling life-stage-specific instantaneous mor-
tality rates. an application to northern anchovy.
Ew
graulis mordar,
eggs and larvae. Fish.
Bull.
U.S.
84:395407.
Peterman, R. M., M.
J.
Bradford,
N.
C.
H.
Lo,
and R.
D.
Contribution of early life stages to interannual
variability in recruitment of northern anchovy
(Engraulis mordax).
Can.
J.
Fish. Aquat. Sci.
45(1):%16.
Picquelle.
S.
J.,
and
R.
P.
Hewitt.
Fish.
Bull.
U.S.
83:137-150.
Methot,
Jr.
1988.
1983.
The northern anchovy spawning biomass
for
the
CalCOFI Rep.
The
1983
spawning biomass of the northern
1982-83
California fishing season.
24:16-28.
anchovy. CalCOFI Rep.
2516-27.
1Y84.
Power,
J.
H.
1986.
A model of the drift of northern anchovy,
En-
graiclis
mordar.
larvae in the California Current.
Fish. Bull.
V.S.
4:W3.
Reid,
J.
L.
1988.
Physical oceanography
1947-1987.
CalCOFI
Rep. 2942-65.
Smith,
P.
E.
1981.
Fisheries on coastal pelagic schooling fish.
Iw
R. Lasker (editor). Marine fish larvae: morphology,
ecology-. and relation to fisheries. p.
1-30.
Univ.
Wash. Press.. Seattle.
Smith,
P.
E.,
and H.
G.
Moser.
1988.
CalCOFI time series: An overview of fishes.
CalCOFI Rep.
2966-78.
Smith,
P.
E.,
and
S.
Richardson.
1977.
Standard techniques for pelagic fish egg and
larva surveys. F.A.O. Fish. Tech. Pap.
175, 100
p.
Taggart,
C.
T., and
W.
C.
Leggett.
198i.
Short-term mortality in post-emergent larval
capelin
Mallolzis
virllosus.
I. Analysis of multiple
in
situ
estimates. Mar. Ecol. Prog. Ser.
41,
p.
205-217.
Changes in body measurements of larval north-
ern anchovy.
Engmulis
mordar.
and other fishes due
to handling and preservative. Fish.
Bull..
U.S.
7868j692
Theilacker,
G.
M.
1980.
Zweifel,
J.,
and
R.
Lasker.
1976.
Prehatch and posthatch growth of fishesa gen-
eral model. Fish. Bull.,
U.S.
745094322,
Zweifel,
J.
R., and
P.
E.
Smith.
1981.
Estimates of the abundance and mortality of
larval anchovies
(1951-1975):
application of a new
method. Rapp. P.-v. Reun. Cons. int. Explor. Mer
i78:24~-259.
to
calculate the mortality
of
young anchovies;
James Zweifel, who developed the methods to do
it; two referees for their critical reviews; and
most of all Dr. Reuben Lasker, who encouraged
all
of
us.
LITERATURE CITED
Ahlstrom.
E.
1948.
A record of pilchard eggs and larvae collected
C.S.
Fish.
Distribution and abundance of egg larvae popula-
C.S.
Fish Wildl. Serv.,
during surveys made in
1939
to
1941.
Wild. Serv. Spec. Sci. Rep.
54,
82
p.
tions of the Pacific sardine.
Fish.
Bull.
93:83-140.
1954.
Bissel, A.
F.
1972.
Butler,
J.
1987.
A negative binomial model with varying element
sizes. Biometrika
59435441.
Comparison of the larval and juvenile growth
and larval mortality rates of Pacific sardine and north-
ern anchovy and implications for species interac-
tion. Ph.D. Thesis. Univ. California at San Diego.
242
p.
Fiedler.
P.
C.,
R.
D.
Methot, and
R.
P. Hewitt.
1986.
Gulland,
J.
A.
Effects of California El Nina
1982-1984
on the
northern anchovy.
J.
Mar. Res.
44:31i438.
1971.
Ecological aspects of fishery research. Adv.
Ecol. Res.
7;115-176.
Hewitt, R.
P.
1981.
The value of pattern in the distribution of young
Rapp.
P.-v.
Reun. Cons. int. Explor. Mer
Spatial pattern and survival of anchovy larvae:
Ph.D.
Historical review
of
the oceanographic approach
fish.
178229-236.
implications of adult reproductive strategy.
Thesis, Univ. California at San Diego.
187
p.
to fishery research.
1982.
1988.
CalCOFI Rep.
29:27-41.
Hewitt,
R.
P., and
G.
D.
Brewer.
1983.
Piearshore production of young anchovy.
CalCOFI Rep.
24:23&244.
Hewitt. R. P., and R.
L).
Methot,
Jr.
larvae in
1978
and
1979.
1982.
Hjort,
J.
1913.
Distribution and mortality of northern anchovy
CalCOFI Rep.
23:226-245.
Fluctuations in the great fisheries of northern
Europe viewed
in
the light of biological research.
Rapp. P.-v. Reun.
Cons.
int. Explor. Mer
19:l-228.
Lenarz,
W.
H.
1972.
Mesh retention
of
Sardiirops
eaerulea
and
Fish. Bull.
Engradis mordar
by plankton nets.
U.S.
70:839-&18.
Lo,
N.
C.
H.
1983.
Re-examination
of
three parameters associated
414

LO
ET.
AI...
ESTIMATES
OF
LARVAL
NO1:TALITY
APPENDIX
Assignment
of
Larval Ages Using the Pareto Function
the integral of the production’curve (Fig.
5)
over these ages:
The standing stock
(SS)
of larvae, between the ages oft,, and
20
days,
is
r
20
r
20
=
[ln(20)
-
In(th)]thPh
for
p
=
1.
Similarly the number of larvae younger than age
t
is
The proportion of larvae that are younger than age
t
is
for
p
=
1.
where
0
<
r(t)
<
1.
By rearranging terms,
t
can be expressed as
t
=
t,,[l
-
r(t
)(1
-
(2O/t/,)-‘h-l)]-I,(h-l) forb
<>
1,
=
th(20/f/,)d‘) forb
=
1,
where
b
is a normal random variable with mean
=
p
and standard error
=
0.2
p
(0.2
is
an arbitrarily chosen value for the coefficient of variation
(b)
and
r(t)
is
a uniform random variable between
0
and
1).
Sample Allocation
The allocation of tows
to
each region and month was based on the
1984
sampling pattern (Table 2, Fig. 2). For a total of
N
tows, the number of
positive tows allocated to month
i
and region
j
was computed
as
n(i,j)
=
N
*
p(i)
*
q(jli)
*
r(jli)
where
p(i)
is the proportion
of
tows for month
i
and
Zp(i)
=
1
q(jli)
is the proportion of tows made in region
j
during month
i
and
r(j)i)
is the proportion of positive tows for region
j
during month
i
Zq(ili)
=
1
and
0
<
=
*li)
<
=
1.
415

FISHERY
BCLLETIS.
YOL.
si.
NO
8.
1489
A uniform random number generator was used to assign each tow to a
month and region. to determine whether the
totv
was positive or not, and
thus to produce
v(i,j).
Assignment
of
the Time
of
Tow
Table
3
lists parameters for Gamma distributions fitted to the actual time
between positive tows minus the minimum time between any two positive
tows (i.e., plankton tows which caught at least one anchovy larva) in each
region because the Gamma distribution takes all values to be greater than
zero. Each distribution is shifted to the right by the constant listed (the
minimum time between any two positive tows). Actual times greater than
150
hours were assumed to be periods of transit to and from port and were
thus excluded when fitting the distributions.
The time of the first tow of a simulated survey was chosen randomly and
incremented by time intervals selected from the distributions described in
Table
3.
If the selected time interval was greater than
4
hours, tows with
zero catch were inserted. The number of zero tows inserted was the time
interval between two positive stations divided by the average travel time
between stations
(2
hours).
416
