
EURASIA Journal of Mathematics, Science and Technology Education, 2019, 15(7), em1731
ISSN:1305-8223 (online)
OPEN ACCESS Research Paper https://doi.org/10.29333/ejmste/103356
© 2019 by the authors; licensee Modestum Ltd., UK. This article is an open access article distributed under the
terms and conditions of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/).
kmageswary@usm.my (*Correspondence) [email protected]om
Addressing Alternative Conceptions about Transition Metals
among Form Six Students using Information and Communication
Technology based Instruction
Mageswary Karpudewan
1*
, Nilavathi Balasundram
1
1
School of Educational Studies, Universiti Sains Malaysia, MALAYSIA
Received 30 November 2018 ▪ Revised 16 January 2019 ▪ Accepted 24 January 2019
ABSTRACT
This study was conducted to reduce the incidence of alternative conceptions about
transition metals among form six students. A quasi-experimental design was carried
out involving 79 students from two intact classes that were randomly classified as the
treatment (N=47) and the comparison (N=32) groups. Information and communication
technology-based instruction (ICT) was used in this study with the treatment group
while a traditional teaching method was used with the comparison group. For the
quantitative study, nine two-tier items were administered in a conceptual test after
instruction to the 79 form six students in both the groups to ascertain their
understanding about transition metals. Five students from the treatment group were
selected for interviews before and after the instruction to obtain further insights into
their understanding. An independent samples t-test analysis was used to compare the
total scores of the two groups in the transition metals conceptual test. The outcome
revealed that there were statistically significant differences in the test mean scores
between the comparison and treatment groups (Mtre = 8.47; SDtre = 0.69; Mcomp =
3.91; SDcomp = 0.96; t = 23.103, p < 0.001). The results from the analysis indicated that
students from the treatment group
showed significantly greater levels of achievement
than the students from the comparison group. Furthermore, the percentage of
alternative conceptions among students in the treatment group was lower than those
of the comparison group students.
Keywords: alternative conceptions, form six students, ICT-based instruction, transition
metals
INTRODUCTION
Almost all science topics including the study of transition metals involve the incidence of alternative conceptions
among students. The study of transition metals is covered in inorganic chemistry in Form 6 (18 to 19 years old) in
the Malaysian education system. This study focuses on alternative conceptions held by students about transition
metals. ICT-based education is about incorporating technology across the curriculum by transferring knowledge,
facts and information using ICT tools such as computers, lap tops, projectors, hand-held devices and more
(Anderson, 2008). It was envisaged that the alternative conceptions about transition metals that are held by students
could be reduced using ICT-based instruction.
Transition metals were first introduced to students in the Periodic Table topic in the first school term. Students
would then learn about transition metals in detail in inorganic chemistry, in the second term. The transition metals
topic in Form 6 covers physical properties and chemical properties of the first row of transition metals such as their
nomenclature, the formation of different colours in transition metal compounds, the ability to form different
oxidation states, formation and bonding of complex ions, transition metals as good catalysts and their uses.

Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
2 / 14
THEORETICAL BACKGROUND
The main objective of science education is to teach science concepts meaningfully to make students aware of
how to use these concepts in their daily lives. Ausubel (1968) argued that meaningful learning would occur if new
concepts are compatible with the old concepts. It is important to know what prior knowledge students bring to the
classroom in order to help them build new concepts (Tsai, 2000).
Chemical knowledge seems to be difficult because it is learned at three levels which are “sub-microscopic,”
“macroscopic” and “symbolic” (Johnstone, 1991). The macroscopic level is something that is physical and visible,
for example, students are able to see the colours of the transition metal compounds. Formation of variable oxidation
states in terms of energies of the 3d and 4s orbitals are at the sub-microscopic level which refers to what is molecular
and invisible. The symbolic level involves equations and chemical symbols such as formation of complex ions with
different types of ligands. Alternative conceptions exist because of the difficulty in comprehending a topic. Thus,
alternative conceptions will indirectly hinder the acquisition of the new knowledge. This is similar to the suggestion
by Özmen (2004) who agrees that alternative conceptions will delay students’ learning of subsequent concepts. In
addition, if students’ preconceptions do not match with scientifically acceptable concepts, they may lack the
necessary basic ideas to build further knowledge that is needed to understand more advanced concepts (Mulford
& Robinson, 2002).
Science education research has shown that children as well as adolescents have their own ideas and concepts
about their surroundings and environments. For instance, Nieswandt (2001) stated that children develop their own
ideas about nature and everyday life at a very early stage. According to Kao (2007) the sources of the students’
alternative conceptions are diverse and are derived from their school teachers, experiments, textbooks, life
experiences, and the use of anthropomorphism, analogies, and intuition. Alternative conceptions reflect situations
in which students provide mistaken explanations to events about their daily experiences.
Several studies have been conducted to investigate the alternative conceptions that students hold in various
science topics. These topics include:
(1) Chemical bonding (Coll & Treagust, 2003; Taber & Coll, 2002), heat, temperature and energetics of chemical
reactions (Goedhart & Kaper, 2002);
(2) Conservation of matter, balancing of equations and stoichiometry (Agung & Schwartz, 2007); (3) acid
strength in organic chemistry (McClary & Bretz, 2012);
(4) Chemical thermodynamics (Hadfield & Wieman, 2010; Sözbilir, Pinarbasi, & Canpolat, 2010);
(5) Solutions, solubility of salts and the particulate nature of salts in solutions (Smith & Nakhleh, 2011);
(6) Acid-base chemistry (Artdej et al., 2010; Drechsle and Schmidt, 2005; Furió-Más, Calatayud, & Bárcenas,
2007).
(7) Transition metals (Sreenivasulu & Subramaniam, 2014)
However, studies rarely have investigated high school students’ understandings about transition metals.
The incidence of alternative conceptions can be reduced using ICT-based instruction to enable students to relate
theory with practice easily. Hu, Gong, Lai & Leung (2018) supported that ICT benefits students learning science in
wide range of topics. Teachers usually use technology-based instruments to reduce alternative conceptions about
transition metals. Using ICT-based instruction will give students a better picture of the concepts compared to
traditional methods of teaching. In fact, teaching and learning assisted with ICT has been increasing worldwide
with its greater accessibility (Baytakar, 2000). Yushau, Mji and Wessels (2003) reported that visual representations
on a computer screen are more beneficial to the students’ understanding as compared to diagrams in books.
A study conducted by Sreenivasulu and Subramaniam (2014) revealed that transition metals is one of the topics
from chemistry that is difficult to comprehend among the students. This is because of the abstract concepts that
Contribution of this paper to the literature
• To this end literature on science education have documented about students developing alternative
conceptions during the science lessons. However, very few studies reported on alternative conceptions
about transition metals.
• The paper illustrates on using ICT based instruction in addressing alternative conceptions on transition
metals. Many studies have employed different approaches in addressing alternative conceptions. This study
specifically illustrates on using ICT in learning about transition metal.
• The ICT based instruction introduced in this study serves as a guide and exemplary for teachers to adapt
the method in teaching about transition metal.

EURASIA J Math Sci and Tech Ed
3 / 14
exist in chemistry especially about transition metals such as metallic bonding, d-d transitions of electrons which
emit colours, formation of complex ions, ligands and so on. Deficiency in understanding the chemistry of transition
metals results in students developing alternative conceptions about the topic. A study by Sreenivasulu and
Subramaniam (2014) identified several alternative conceptions about transition metals. These included: transition
metals with zero oxidation state cannot attract ligands to form complex ions, Cu
+
has unpaired d-electrons which
can take part in d-d transitions, Cu and K have the same ionisation energy for the loss of their 4s electrons due to
similar screening effects, etc. Furthermore, Johnstone (1971) found that students have difficulty in conceptualizing
how the splitting of d-orbitals occurs in transition metals. He also reported that knowledge of d-orbitals is the basic
knowledge that a student should acquire in order to learn about splitting of d-orbitals.
ICT-based Instruction
In recent years, the use of ICT has been widespread because the use of computers and other electronic forms of
media in science teaching saves time and helps students better understand science concepts (Lai, Hwang & Tu,
2018). Most teachers and experts recognize the need for a teaching method using ICT facilities. Adam and Tatnall
(2010) believe that ICT has positive impact on students’ understanding of science concepts while Barak (2017) has
suggested that ICT-based instruction plays a crucial role in current teaching and learning of science concepts.
Computers are used as an additional tool in schools to achieve educational goals (Bayraktar, 2000). Education in
the 21
st
century that involves the use of ICT in the classroom plays a vital role in enhancing the teaching and learning
process. The role of ICT is to help students in particular, to learn and teachers to perform their teaching more
effectively (Goktas & Yildrim, 2003). Integrating computers into the teaching and learning of inorganic chemistry
can go a long way in solving the above problem, as it is an alternative approach that is available to teachers and
students in the teaching and learning of chemistry (Anderson, 2002; Gyöngyösi, 2005).
The major propositional content knowledge statements covered in transition element instruction using ICT are
shown in Table 1.
PURPOSE OF THE STUDY
This study was conducted with the purpose of reducing the incidence of alternative conceptions about transition
metals among Form 6 students. The study was guided by the main research question (RQ): What is the effect of
ICT-based instruction on reducing the incidence of alternative conceptions about the chemistry of transition metals
among Form 6 students?
Table 1. Major propositional content knowledge statements defining instruction on transition metals
Item
No.
Propositional content knowledge statements
1.
A transition metal in zero oxidation state is able to attract ligands and form metal complexes, e.g., Ni in oxidation state
zero forms nickel (0)tetracarbonyl, Ni(CO)
4
.
2.
The tendency for transition metals to involve all 3d electrons in bonding will decrease once the d
5
configuration is
exceeded because the electrons will pair up and will not be available for bonding.
3.
Cu
+
has unpaired d electrons which cannot take part in d-d transitions because the electronic configuration of Cu
+
is [Ar]
3d
10
4s
0
, so it has completely filled 3d orbitals.
4.
Cu and K do not have the same ionisation energy for the loss of their 4s electrons because they do not have the same
screening effect of their inner electrons.
5.
The reactivity of transition metals decreases from left to right across a period because the d orbitals are progressively
filled up with electrons.
6
The ionisation energy of transition metals down a group in the Periodic Table is not similar to that of the alkali metals
because the screening effect of the inner electrons of transition metals increases.
7.
Transition metal ions are the only ones that are coloured as ions of non-transition metals like sodium, calcium, sulfide,
chloride, iodide and bromide ions are colourless.
8.
Transition metals can be good reducing agents because they have relatively low standard electrode potentials, e.g. E
o
of
Sc
3+
/Sc = -2.08.
9.
Transition metals are good catalysts because they have empty or partially filled d-orbitals that can be used to form
temporary bonds with reactant molecules.

Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
4 / 14
METHODS
Sampling and Research Design
This study was aimed at reducing the alternative conceptions about transition metals among form six secondary
school students. For this purpose, a quasi-experimental design, involving 79 form six (18 to 19 years old) science
stream students was used in this study. The students were divided into two groups where one class of 32 students
(referred to as the comparison group) was taught using a traditional method. The other group of 47 students from
two classes (referred to as the treatment group) was taught using ICT-based instruction. Form Six is pre-university
level studies. After completing Form Six students will be enrolled into degree courses of their choice. Chemistry is
a requirement for students to enroll into science, technology, engineering and mathematics courses at tertiary level.
Research Instrument
Both quantitative and qualitative research methods were used in this study. A conceptual test was administered
to both groups after the treatment. As the students were introduced to the chemistry of transition metals for the
first time, it was not considered necessary to administer a pretest. In this test, true-false questions were
administered to students to identify alternative conceptions that were held by them. This conceptual test that was
adapted from a study by Sreenivasulu and Subramaniam (2014), consisted nine multiple-choice true-false
questions. Each item was followed by an open-ended question that required students to give a reason for the answer
chosen in the item. Items in the conceptual test are listed in Figure 1 together with the expected reasons In addition,
an interview session was conducted with the Form 6 students from the treatment group, before and after
instruction, to further enhance the results of this study.
Name: ______________________________________________________________________
Class: ____________________
Answer each question by choosing A for correct statement and B for incorrect statement. Provide a reason for each chosen statement.
No.
Question
1
A transition metal in zero oxidation state cannot attract ligands and hence cannot form complexes.
A. True *B. False
Expected reason: Transition metal with zero oxidation state can attract ligands because it has empty orbitals to be occupied by
ligands. For example, nickel in oxidation state zero, forms Ni(CO)
4
.
2.
The tendency for transition metals to involve all 3d electrons in bonding will not decrease once the d
5
configuration is exceeded.
A. True *B. False
Expected reason: Once the d
5
configuration is exceeded the electrons will pair up and will not be available for bonding.
3.
Cu
+
has unpaired d electrons which can take part in d-d transitions.
A. True *B. False
Expected reason: The electronic configuration of Cu
+
is [Ar]3d
10
4s
0
, so has completely filled 3d orbitals and cannot take part in d-d
transitions.
4.
Cu and K are expected to have the same ionisation energy for the loss of their 4s electrons.
A. True *B. False
Expected reason: Cu and K do not have the same screening effect of their inner electrons.
5.
The reactivity of transition metals decreases from left to right across a period.
*A. True B. False
Expected reason: The d orbitals are progressively filled up with electrons.
6.
The ionisation energy of transition metals down a group in the Periodic Table is similar to that of the alkali metals.
A. True *B. False
Expected reason: The screening effect of the inner electrons of transition metals increases.
7.
Only transition metal ions are coloured.
A. *True B. False
Expected reason: Only transition metals undergoes d-d orbitals splitting to form coloured ions. Other ions of non-transition
elements like sodium, calcium, sulfide, chloride, iodide and bromide ions are colourless.
8.
Transition metals can be good reducing agents.
*A. True B. False
Expected reason: They have relatively low standard electrode potentials, e.g. E
o
of Sc
3+
/Sc = -2.08.
9.
Transition metals are good catalysts.
*A. True B. False
Expected reason: They have empty or partially filled d-orbitals that can be used to form temporary bonds with reactant molecules.
(* correct answer)
Figure 1. Questions included in the conceptual test
EURASIA J Math Sci and Tech Ed
5 / 14
Treatment
Nine periods a week were allocated for chemistry lessons and three periods were allocated for laboratory
sessions together with the students’ project work. Each period consisted of forty minutes of lesson time.
Traditional Teaching Method
The traditional teacher-centered method was used to teach transition metals to the comparison group. A white
board, textbooks and additional notes were used in the classroom by the teacher. This instruction was more chalk-
and-talk in nature where the students listened passively to the teacher and took down notes when necessary. This
was followed by discussion of examples and a few exercises from the textbook. The teacher administered the
conceptual test to identify the alternative conceptions at the end of the instruction.
ICT-based Instruction
ICT-based instruction was used with the treatment group. The teacher used the teaching courseware on a CD
provided by the Ministry of Education. This courseware comprised of lessons in each topic as well as practical
activities and problems that the students had to solve. During the lessons the students were shown the formation
of complex ions, d-d transitions, etc. using the CD. This was followed by a group activity after each lesson. For
example, after students had viewed the video of the formation of complex ions, they were required to form their
own complex ion using a different central metal ion and ligands. A representative from each group then explained
their complex ion to the class. The teacher then gave her opinions and comments on the students’ presentation.
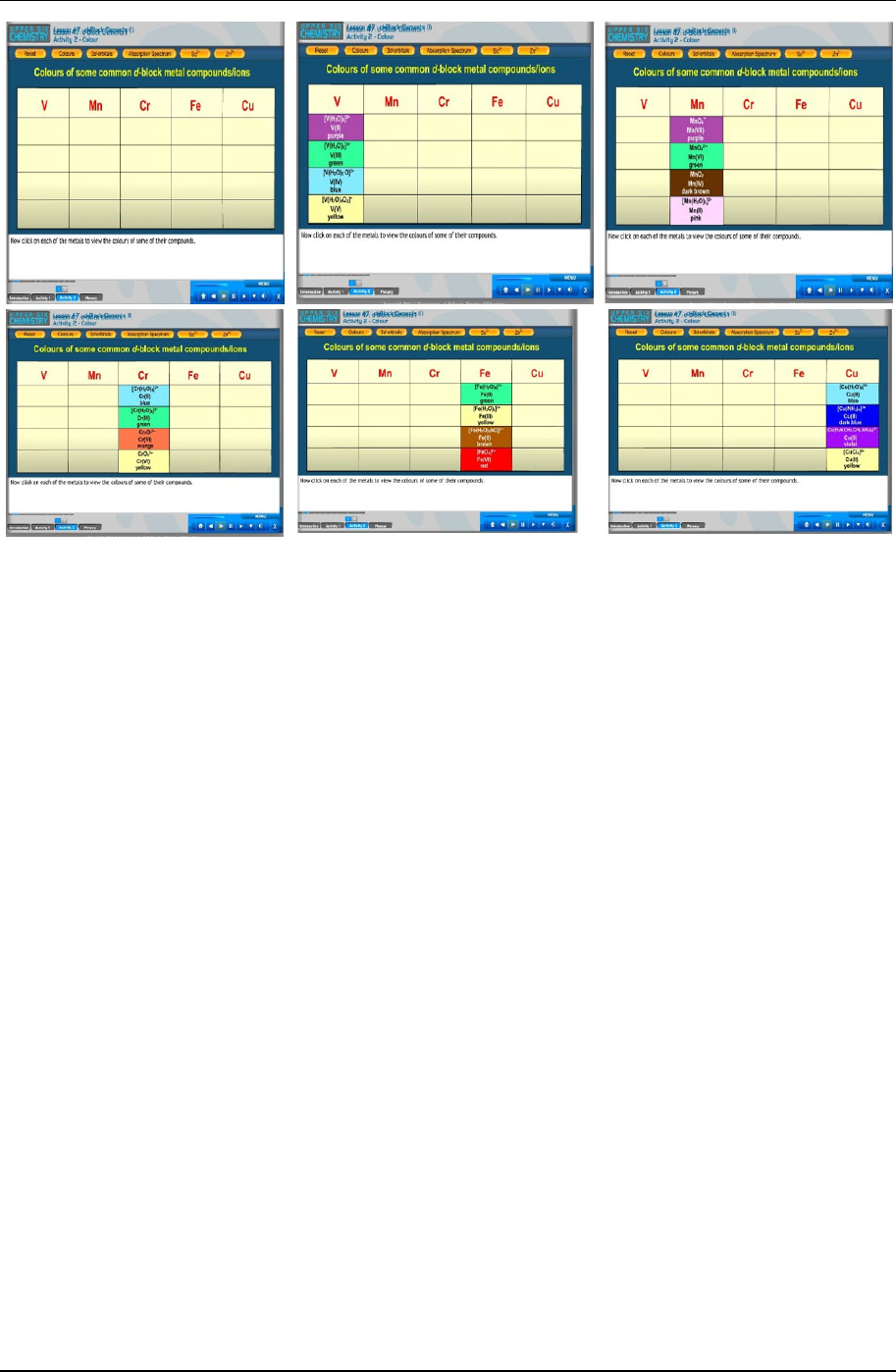
Figure 2 shows several screen shots from the CD that the teacher could use to show the colours of the ions of
various transition metal ions.
Figure 2. Shows a series of screen shots that the teacher could use to show that the colours are due to d electron transitions
Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
6 / 14
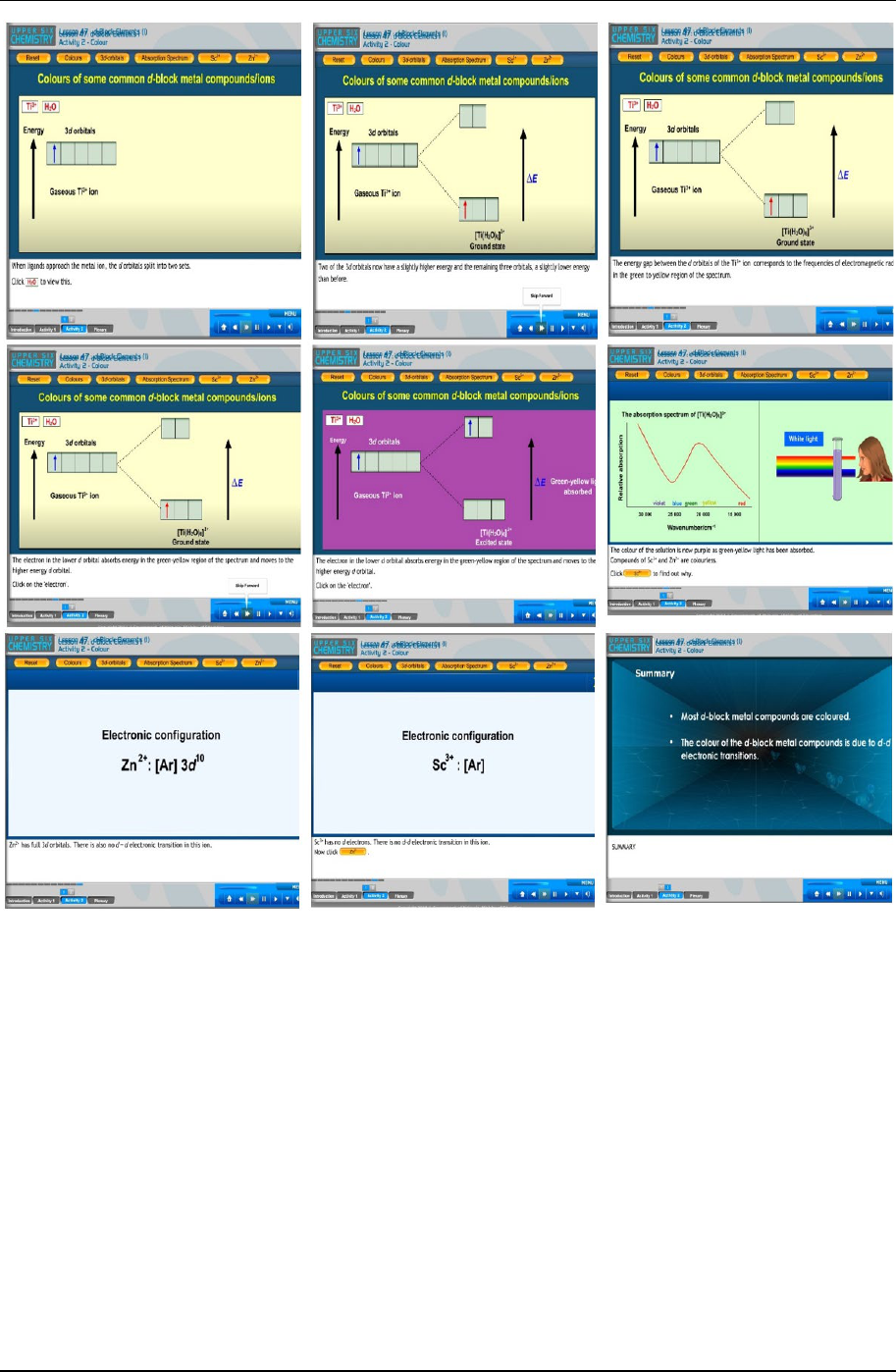
To further enhance their knowledge about the formation of complex ions, the students carried out laboratory
work. It helped students to have a clearer picture about the concepts and enhanced their understanding about the
transition metals topic. Students were shown examples of complex ion solutions (e.g., Ni (NH
3
)
6
2+
and Cu(NH
3
)
4
2-
) to emphasize the various colours of the solutions (see Figure 4).
Figure 3. Colours of ions due to d electron transitions

EURASIA J Math Sci and Tech Ed
7 / 14
The teacher gave students exercises after each sub-topic to do as homework.
Data Collecting Method
An item was considered correct when only both parts of items were correctly answered (Treagust, 1988). No
marks were awarded for any other combination of answers. Interviews were conducted with five students from
the treatment group who were randomly selected by the first author, to ascertain the extent of their alternative
conceptions before and after instruction. There was no time limit for the interview sessions.
Data Analysis
For the quantitative results, the posttest data of the comparison and treatment groups were analyzed using SPSS
21.0 (Statistical Package for the Social Sciences). For the qualitative results, five interview questions were used in
interviews to ascertain the treatment group students’ understandings about transition metals before and after
instruction.
RESULTS
The results of quantitative and qualitative analysis of the data are reported below.
Comparison of the Total Scores in Transition Metals Posttest
The transition metals conceptual test was administered to the students from the comparison group and
treatment group after the treatment had been carried out. Independent samples t-test analysis showed statistically
significant differences in the test mean scores between the comparison and treatment groups (see Table 2). These
results show that the students in the treatment group exhibited improved understanding about transition metals
compared to the students from the comparison group.
Figure 4. Examples of coloured complex ion solutions
Table 2. Themes formed after the reviewing and refining process
No.
Initial theme
Theme
1.
The oxidation number of transition metals ions
Nature of transition metal ions to form
complex ions
2.
Bonding in the complex ion
3.
Comparison of ionization energy between copper and potassium
Comparison of ionization energy between
alkali metals and transition metals
4.
Comparison of the trend in ionization energy between alkali metals and
transition metals
5.
Comparisons of color between transition metal ion and other ions
Colors in transition metal ions
6.
The explanation for some transition metal ions which are not colored
7.
Reactivity trend of transition metals across the period
Chemical reactions of transition metals
8.
The strength of transition metals as reducing agents

Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
8 / 14
Students’ Alternative Conceptions about Transition Metals
The objective of this study was to reduce the alternative conceptions about transition metals among Form 6
students using ICT-based instruction. The two-tier questions in the conceptual test evaluated students’
understanding in four areas which are formation of complex ions, variable oxidation states in transition metals,
ionisation energy of transition metals, formation of colours in transition metal ions and uses of transition metals.
The alternative conceptions that were identified are shown in Table 3.
As shown in Table 3, several alternative conceptions were identified about transition metals through the
responses given by the students using the two-tier questions. There is a large difference in the percentage of the
alternative conceptions between the comparison group and the treatment group students. The percentage of
alternative conceptions held by the comparison group students ranged from 44% to 72%, while for the treatment
group students the percentage of alternative conceptions ranged from 2% to 11%. This clearly shows that ICT-based
instruction had resulted in a significantly lower incidence of alternative conceptions among the treatment group
students compared to the traditional instruction with the comparison group. For qualitative data, the same five
interview questions were used before and after instruction to determine the understanding about transition metals
of the treatment group students. The questions that were used in the interviews involved the following areas:
(1) The formation of complex ions
(2) Variable oxidation states of the transition metals
(3) Ionisation energy of transition metals
(4) Formation of coloured compounds by transition metal ions
(5) Uses of transition metals
Formation of Complex Ions
Based on the quantitative analysis of the results of the conceptual test, 50% of the students from the comparison
group and 2% of the students from the treatment group held the alternative conception that a transition metal in zero
oxidation state cannot attract ligands and hence cannot form complexes. The alternative conception had decreased from
the comparison group to the treatment group. Students understood better after demonstrating the formation of
complex ions using ICT technology rather than traditional teaching. Students could observe that transition metals
have empty orbitals that can be filled by the electrons from the ligands even without donating any of the electrons.
The interview responses below show students’ understanding of transition metals having zero oxidation state
before the instruction took place.
Student 1:
Transition metals have various oxidation states and it is one of their special properties.
Student 2:
They should have more than one oxidation state like Cu has +1 and +2 oxidation states.
Student 3:
Transition metals can donate more than one electron to form various oxidation states.
Student 4:
All metals have oxidation states.
Student 5:
Don’t know………Maybe they can because if it is a solid then it will have zero oxidation state.
Based on students’ responses, it may be concluded that all the five students had some level of alternative
conceptions about the formation of zero oxidation state by transition metals. Student 5 was unable to relate the
oxidation number to a solid or ion. Students 1, 2, 3 and 4 basically knew that transition metals form various
oxidation states.
After ICT-based instruction, the same students were interviewed using the same.
Student 1:
Transition metals such as Ni(CO)
4
can have zero oxidation state. Transition metals have various
oxidation states including zero oxidation state.
Student 2:
Transition metals can form complex ions with zero oxidation state.
Student 3:
Ni(CO)
4
has zero oxidation state in its compound.
Student 4:
Transition metals can have zero oxidation state. For example, nickel in Ni(CO)
4
has zero oxidation state.
Student 5:
Zero oxidation state can occur in transition metal compounds.
Table 3. Means and standard deviations for the results of the posttest
Test
Treatment group (N = 47)
Comparison group (N = 32)
t p
Mean
SD
Mean
SD
Post-test
8.47
0.69
3.91
0.96
23.103
< 0.001

EURASIA J Math Sci and Tech Ed
9 / 14
Based on the students’ answers, it may be concluded that all the students had already overcome the alternative
conception on variable oxidation state in transition metals. All the students agreed that transition metals can form
zero oxidation state. Students 1, 3, and 4 could include the example of Ni(CO)
4
as having a transition metal with
zero oxidation state.
Variable Oxidation States
There were three alternative conceptions that were identified about the variable oxidation states of transition
metals. As many as 59% of the students from the comparison group and 6% of the students from the treatment
group believed that the tendency for transition metals to involve all 3d electrons in bonding will not decrease once the d
5
configuration is exceeded. When the d
5
configuration is exceeded, the electrons will start to pair up and there will be
less tendency to involve electrons from the d orbitals to form bonds. The CD provided by the Ministry of education
helped students to understand that when the d orbital is half-filled, the electrons will start to pair up and will
reduce the formation of bonds.
Interview responses below show that before the ICT-based instruction, the involvement of the electrons in
bonding in transition metals decreases once the d
5
configuration is exceeded.
Student 1:
Metals form ionic bonds. Hmmm…I don’t think so because transition metals need to donate higher
number of electrons to form ionic bonds.
Student 2:
No, because transition metals have many empty orbitals. They can fill the empty orbitals such as 4s, 4p
and 4d orbitals to form bonds.
Student 3:
Hmmm….Transition metals with higher number of electrons cannot form bonds with the ligands
because higher energy is needed to donate all the electrons.
Student 4:
To donate more electrons, more energy is needed.
Student 5:
Hmm…not sure…but I think transition metals are reactive because they are catalysts for most reactions.
So they can form more bonds.
It may be concluded that students are not very sure about bond formation after the d
5
configuration is exceeded.
Students 1, 3 and 4 held the alternative conception that transition metals can only form ionic bonds. Student 2 held
the alternative conception that the electrons can be expanded to 4s, 4p and 4d orbitals. Student 5 held the alternative
conception that the transition metals are reactive.
The interview responses below show the students’ understanding after ICT-based instruction was carried out.
Student 1:
Yes, it decreases because after the d
5
configuration has exceeded, the electrons will start to pair up and
will not be available for bonding.
Student 2:
It decreases because electrons will pair up after the d
5
configuration has been exceeded.
Student 3:
Once the electrons pair up in each orbital, they will not be available for bonding.
Student 4:
Involvement of the electrons for bonding in transition metals decreases because the number of unpaired
electrons also decreases after the d
5
configuration is exceeded.
Student 5:
Involvement of the electrons for bonding in transition metals decreases because electrons will pair up in
each d orbital.
Based on the students’ answers it may be concluded that most of them had overcome the alternative conception.
All of them knew that the tendency for transition metals to involve all 3d electrons in bonding will decrease once
the d
5
configuration is exceeded because the electrons will pair up and will not be available for bonding.
Reactivity of Transition Metals
Another alternative conception held by students was that the reactivity of transition metals increases from left to
right across a period. Reactivity of the transition metals decreases as there is a decrease to involve the electrons from
the d orbitals to form bonds. Fifty percent of students from the comparison group held this alternative conception
but only 9% of the students held this alternative conception in the treatment group, showing a decrease in the
alternative conception held by the students in the treatment group compared to comparison group.
Transition Metals as Reducing Agents
A total of 63% of students from the comparison group believed that transition metals are not good reducing agents.
However, this alternative conception had decreased to 4% of the students in the treatment group.

Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
10 / 14
Ionisation Energy of Transition Metals
Students held the alternative conception about the ionisation energy of transition metals compared to the
ionisation energy of s-block metals with 53% of the students from the comparison group holding the alternative
conception that the ionisation energy of transition metals down a group in the Periodic Table is similar to that of the alkali
metals. Only 4% from the treatment group believed similarly.
Also, 63% of students from the comparison group held the alternative conception that Cu and K are expected to
have the same ionisation energy for the loss of their 4s electrons while only 9% of the treatment group students held this
alternative conception. This alternative conception arose due to the poor understanding of the students about the
trend in the ionisation energy of transition metals and s-block metals.
Result from the interview before instruction about whether copper and potassium have the same ionization
energy:
Student 1:
hmm…I don’t think so.
Student 2:
They have the same ionisation energy.
Student 3:
No….
Student 4:
Hmmm…not the same I think.
Student 5:
Can be the same…
Responses of students that copper and potassium have same or different ionization energy are given below:
Student 1:
Because they are different elements with different electronic configurations, different amount of energy
is needed to remove the electron.
Student 2:
Because potassium and copper are in the same row in the Periodic Table. So they can have the same
ionisation energy.
Student 3:
Potassium is a group 1 metal and copper is a transition metal. So the ionisation energy must be different.
Student 4:
Both are metals and can donate electrons easily. Therefore, their ionisation energy can be the same with
the same oxidation number.
Student 5:
Because both are metals and both can form Cu
+
and K
+
, both involve the removal of one electron.
Based on the students’ answers it may be concluded that most of the students (Student 2, 3, and 4) held
alternative conceptions. Student 2 held the alternative conception that if metals are in the same row they have the
same ionisation energy. Student 4 held the alternative conception that metals can donate electrons, therefore, they
have the same ionisation energy. Student 5 held the alternative conception that if the oxidation number is the same,
the metals are most likely to have the same ionisation energy. Students understood the term ionisation energy but
they could no explain the trend of the ionisation energy. Students 1 and 3 believed that they were different metals
and so they have different ionisation energies.
Responses from interviews after ICT-based instruction are as follows:
Student 1:
The ionisation energy of potassium is lower than that of copper. The first electron is being removed from
the 4s orbital of copper and potassium. The size of the copper atom is smaller than that of the potassium
atom. The nuclear charge of copper is also higher than that of potassium. In conclusion, the 4s electron
in copper is more strongly held by the nucleus and so is more difficult to be removed.
Student 2:
The ionisation energy of potassium is lower than that of copper because the atomic size of potassium is
larger than that of copper. Therefore, the valence electrons in copper are more strongly held by the
nucleus compared to potassium. More energy is need to remove the valence electron in copper compared
to potassium.
Student 3:
: It doesn’t have the same ionisation energy. Potassium has a larger size than copper. Potassium has
lower nuclear charge than copper. Thus, the ionisation energy for potassium is lower than copper.
Student 4:
Potassium with larger size and lower nuclear charge would be expected to have lower ionisation energy
than copper. Less energy is needed to remove an electron from potassium compared to copper.
Student 5:
Copper and potassium don’t have the same ionisation energy. This is because the energy needed to
remove an electron from copper is higher than potassium. Copper has a smaller atomic size than
potassium and larger proton number than potassium. More energy is needed to remove an electron from
copper compared to potassium because the outermost electron in copper has stronger attraction towards
the nucleus compared to potassium. So the ionisation energy in copper is higher than potassium
It may be concluded that all the students had overcome the alternative conceptions only up to a certain extent
after instruction. Only students 1 and 5 had a complete idea by comparing the ionisation energy between a
transition metal and an alkaline earth metal. These students compared the size, nuclear charge, strength of nucleus

EURASIA J Math Sci and Tech Ed
11 / 14
holding the outermost electron and energy needed to remove the electron. Students 2, 3 and 4 gave brief responses
comparing the size and nuclear charge only to describe the ionisation energy.
Formation of Colours in Transition Metal Ions
ICT-based instruction plays an important role in reducing the alternative conception about the formation of
coloured ions of transition metals. The teacher showed the experimental group students the video of d orbitals that
can be split into two energy levels and the promotion of the electrons from a lower energy level to a higher energy
level. Students also managed to see all the shapes of the d orbitals in the video presented as it is difficult to draw
on the board. Thus, students were able to visualize the shapes of the d orbitals and the reason transition metal ions
are coloured. As a result, only 11% of the students from the treatment group held the alternative conception that
Cu
+
has unpaired d electrons which can take part in d-d transitions. In the traditional teaching method, students could
not visualize d orbitals well because the teacher showed the shapes of the d orbitals from the textbook. This resulted
in 53% of students from the comparison group holding an alternative conception. Another prevalent alternative
conception among students was that not only transition metal ions are coloured. Students perceived that there are other
coloured ions as well. Students from the treatment
group understood that any metals that undergo d-d transition
are coloured whereas students from the traditional teaching failed to understand this.
The teacher questioned the students to find out why the Cu
+
ion is colourless.
Student 1:
Hmm… don’t know.
Student 2:
No idea.
Student 3:
Not sure.
Student 4:
All transition metals ions are coloured except for Cu
+
…hmm.
Student 5:
Don’t know.
Based on the students’ answers, it may be concluded that all the students have alternative conceptions about
the formation of the colour of the Cu
+
ion. None of them gave a correct response. Students were not sure of the
reason why the Cu
+
ion is colourless because they assumed that all transition metal ions are coloured.
After ICT-based instruction, students showed positive results in their interview responses.
Student 1:
Because the copper ion has fully filled d orbitals. Thus, Cu
+
cannot undergo d-d transition.
Student 2:
Cu
+
cannot undergo d-d transition. There will be no electrons promoted to a higher energy level to absorb
a certain amount of wavelength to reflect the colours. That’s why it is colourless.
Student 3:
Cu
+
has fully filled d orbitals and there will be no electrons promoted to a higher energy level. There is
no d-d transition and hence Cu
+
is not coloured.
Student 4:
d orbitals are fully filled. Any metals that undergo d-d transition are coloured. All transition metals ions
are coloured except for Cu
+
. So, Cu
+
cannot undergo d-d transition because electrons are not being
promoted to higher energy level.
Student 5:
d orbitals of Cu
+
are fully filled.
None of the electrons from the Cu
+
get promoted to a higher energy level.
So, it is colourless.
It may be concluded that all the students held alternative conceptions before instruction on the formation of the
colour. None of them gave a correct response. Students were not sure of the reason why the Cu
+
ion is colourless
because they have a conception that all transition metal ions are coloured.
Uses of Transition Metals
One alternative conception that students held was that transition metals are not good catalysts. Forty-four percent
of the students from the comparison group misunderstood that transition metals are not good catalysts whereas
only 9% of the students from the treatment group managed to comprehend the correct concept. After the treatment
it was found that students from the treatment group understood that transition metals have empty d orbitals that
could be occupied by the electrons to form temporary bonds. Thus, they can be good catalysts compared to the
other metals. The teacher in the ICT-based instruction helped to improve students’ understanding by showing all
empty orbitals that existed in transition metals with examples.

Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
12 / 14
Based on the post-instruction interviews, students’ understanding about transition metals improved after the
intervention. Students from the comparison group understood the transition metals concepts better than before.
Students displayed alternative conceptions in the formation of complex ions, variable oxidation states of the
transition metals, ionisation energy of transition metals, formation of the colours in transition metal ions and the
uses of transition metals before instruction. However, after the intervention, the incidence of alternative
conceptions had been reduced.
CONCLUSION
This study used a two-tier questionnaire about transition metals to ascertain the alternative conceptions that
existed among form six students. The treatment group used ICT-based instruction to teach the students about
transition metals whereas traditional teaching was used with the comparison group. In response to the research
question of the study (What is the effect of ICT-based instruction on reducing the incidence of alternative
conceptions about the chemistry of transition metals among Form 6 students?), this study showed that ICT-based
education improved students understanding compared to the traditional teaching method. In addition, students
were interviewed before and after the treatment to see if ICT-based instruction had improved their understanding
about transition metals. It was found ICT-based instruction helped the students to reduce the incidence of
alternative conceptions and improved their understanding about transition metals. Tsai and Chou (2002) in their
research found that the achievement of the students increased with the use of computers in science education.
Similarly, Kim et.al. (2013) described ICT as having an important role in improving teaching and learning. This
study will contribute to the improvement of chemistry teaching especially about transition metals. Teachers’
awareness of students’ understanding before
a lesson is very important to help reduce alternative conceptions,
indirectly contributing to the improvement of teaching and achievement of better understanding in a particular
topic.
REFERENCES
Adam, T., & Tatnall, A. (2010). Use of ICT to assist students with learning difficulties: An actor-network analysis.
In N. Reynolds & M. Turcsanyi-Szabo (Eds.), Key competencies in the knowledge society (pp. 1-11). New York,
NY: Springer. https://doi.org/10.1007/978-3-642-15378-5_1
Agung, S., & Schwartz, M. S. (2007). Students’ understanding of conservation of matter, stoichiometry and
balancing equations in Indonesia. International Journal of Science Education 29(3), 1679–702.
https://doi.org/10.1080/09500690601089927
Anderson, J. (2002). Being mathematically educated in the twenty-first century: What should it mean? In L.
Haggarty (Ed.), Teaching Mathematics in Secondary Schools, London, UK: Routledge Falmer.
https://doi.org/10.4324/9781315013152
Anderson, R. (2008). Implications of the information and knowledge society for education. In J. Voogt, & G. Knezek
(Eds.), International handbook of information technology in primary and secondary education. New York, NY:
Springer. https://doi.org/10.1007/978-0-387-73315-9_1
Artdej, R., Ratanaroutai, T., & Coll, R. K. (2010). Thai grade 11 students’ alternative conceptions for acid-base
chemistry. Research in Science & Technological Education, 28(2), 167-183.
https://doi.org/10.1080/02635141003748382
Table 4. The percentage of students’ having alternative conception as determined in the conceptual test after instruction
Items
nos.
Alternative conceptions
Comparison
group (%)
Treatment
group (%)
1
A transition metal in zero oxidation state cannot attract ligands and hence cannot form
complexes.
50 2
2
The tendency for transition metals to involve all 3d electrons in bonding will not decrease once
the d
5
configuration is exceeded.
59 6
3
Cu
+
has unpaired d electrons that can take part in d-d transitions.
53
11
4
Cu and K are expected to have the same ionisation energy for the loss of their 4s electrons.
63
9
5
The reactivity of transition metals increases from left to right across a period.
50
9
6
The ionisation energy of transition metals down a group in the Periodic Table is similar to that
of the alkali metals.
53 4
7
Not only transition metal ions are coloured.
72
6
8
Transition metals are not good reducing agents.
63
4
9
Transition metals are not good catalysts.
44
9

EURASIA J Math Sci and Tech Ed
13 / 14
Ausubel, D. (1968). Educational Psychology: A Cognitive View. Holt, Rinehart and Winston, New York.
https://doi.org/10.3102/00028312005003421
Barak, M. (2017). Science teacher education in the twenty-first century: a pedagogical framework for technology-
integrated social constructivism. Research in Science Education, 47(2), 283-303.
https://doi.org/10.1007/s11165-015-9501-y
Bayraktar, Ş. (2000). A meta-analysis on the effectiveness of computer-assisted instruction in science education
(Unpublished Master Dissertation), Ohio University, US.
https://doi.org/10.1080/15391523.2001.10782344
Coll, R. K., & Treagust, D. F. (2003). Learners’ mental models of metallic bonding: A cross-age study. Science
Education 87(5), 685–707. https://doi.org/10.1002/sce.10059
Drechsle, M., & Schmidt, H-J. (2005). Textbooks’ and teachers’ understanding of acid-base models used in chemistry
teaching. Chemistry Education Research and Practice 6(1), 19-35. https://doi.org/10.1039/B4RP90002B
Furió-Más, C., Calatayud, M. L., & Bárcenas, S. L. (2007). Surveying students’ conceptual and procedural
knowledge of acid-base behavior of substances. Journal of Chemical Education 84(10), 1717–24.
https://doi.org/10.1021/ed084p1717
Goedhart, M. J., & Kaper, W. (2002). From chemical energetics to chemical thermodynamics. In Gilbert, J. K., Jong,
O. D., Justi, R., Treagust, D. F., and Van Driel, J. H. (Eds), Chemical Education: Towards Research-Based Practice.
Kluwer, Dordrecht, The Netherlands, pp.339-362. https://doi.org/10.1007/0-306-47977-x_15
Goktas, Y., & Yildrim, Z. (2003). A comparative analysis of the EU countries’ and Turkey’s regarding the integration
of ICT in primary education curricula and teacher education programs, Retrieved from
http://www.leeds.ac.uk/educol/documents/00003490.htm
Gyöngyösi, E. (2005). Continuing education for mathematics teachers of secondary education to use computers
more effectively and to improve education. Retrieved from
http://www.cimt.plymouth.ac.uk/journal/egcomp.pdf.
Hadfield, L. C., & Wieman C. E. (2010). Student interpretations of equations related to the first law of
thermodynamics. Journal of Chemical Education 87(7), 750–755. https://doi.org/10.1021/ed1001625
Hu, X., Gong, Y., Lai, C., & Leung, F. K. (2018). The relationship between ICT and student literacy in mathematics,
reading, and science across 44 countries: A multilevel analysis. Computers & Education.
https://doi.org/10.1016/j.compedu.2018.05.021
Johnstone, A. D. (1971). The spreading of a proton beam by the atmosphere. Planetary and Space Science, 20(2), 292-
295. https://doi.org/10.1016/0032-0633(72)90111-0
Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer
Assisted Learning 7(2), 75-83. https://doi.org/10.1111/j.1365-2729.1991.tb00230.x
Kao, H. L. (2007). A Study of Aboriginal and Urban Junior High School Students’ Alternative Conceptions on the
Definition of Respiration. International Journal of Science Education, 29(4), 517-533
https://doi.org/10.1080/09500690601073376
Kim, C., Kim, M. K., Lee, C., Spector, J. M., & DeMeester, K. (2013). Teacher beliefs and technology integration.
Teaching and Teacher Education, 29, 76-85. https://doi.org/10.1016/j.tate.2012.08.005
Lai, C. L., Hwang, G. J., & Tu, Y. H. (2018). The effects of computer-supported self-regulation in science inquiry on
learning outcomes, learning processes, and self-efficacy. Educational Technology Research and Development, 1-
30. https://doi.org/10.1007/s11423-018-9585-y
McClary, L. M., & Bretz, S. L. (2012). Development and assessment of a diagnostic tool to identify organic chemistry
students’ alternative conceptions related to acid strength. International Journal of Science Education, 34(5),
2317–2341. https://doi.org/10.1080/09500693.2012.684433
Mulford, D. R., & Robinson, W. R. (2002). An inventory for alternate conceptions among first semester general
chemistry students. Journal of Chemical Education 79(6), 739–744. https://doi.org/10.1021/ed079p739
Nieswandt, M. (2001). Problems and possibilities for learning in an introductory chemistry course from a
conceptual change perspective. Science Education, 85(2), 158-179. https://doi.org/10.1002/1098-
237X(200103)85:2<158::AID-SCE40>3.0.CO;2-3
Özmen, H. (2004). Some Student Misconceptions in Chemistry: A Literature Review of Chemical Bonding. Journal
of Science Education and Technology, 13(2), 147–159. https://doi.org/10.1023/B:JOST.0000031255.92943.6d
Smith K. C., & Nakhleh M. B. (2011). University students’ conceptions of bonding and melting and dissolving
phenomena. Chemistry Education Research and Practice, 12(4), 398–408.
https://doi.org/10.1039/C1RP90048J

Karpudewan & Balasundram / Alternative Conceptions about Transition Metals
14 / 14
Sözbilir, M., Pinarbasi, T., & Canpolat, N. (2010). Prospective chemistry teachers’ conceptions of chemical
thermodynamics and kinetics. Eurasia Journal of Mathematics, Science & Technology Education, 6(2), 111-120.
https://doi.org/10.12973/ejmste/75232
Sreenivasulu, B., & Subramaniam, R. (2014). Exploring undergraduates’ understanding of transition metals
chemistry with the use of cognitive and confidence measures. Research in Science Education, 44(6), 801-828.
https://doi.org/10.1007/s11165-014-9400-7
Taber, K. S., & Coll, R. K. (2002). Bonding. In Gilbert, O. De Jong, R. Justi, D. F. Treagust &J.H. Van Driel (Eds.),
Chemical Education: Towards Research-Based Practice, pp. 213-234. Dordrecht, The Netherlands: Kluwer.
https://doi.org/10.1007/0-306-47977-x_10
Treagust, D. F. (1988). Development and use of diagnostic tests to evaluate students’ misconceptions in science.
International Journal of Science Education 10(2), 159–169. https://doi.org/10.1080/0950069880100204
Tsai, C.-C. (2000). Enhancing science instruction: The use of conflict maps. International Journal of Science Education
22(3), 285–302. https://doi.org/10.1080/095006900289886
Tsai, C.-C., & Chou, C. (2002). Diagnosing students’ alternative conceptions in science. Journal of Computer Assisted
Learning, 18(2), 157–165. https://doi.org/10.1046/j.0266-4909.2002.00223.x
Yushau, B., Mji, A., & Wessels, D. C. J. (2003). Creativity and computer in the teaching and learning of mathematics.
Retrieved from www.kfupm.edu.sa/math/UPLOAD/Tech_Reports/311.pdf
http://www.ejmste.com
