
BRUCELLOSIS REFERENCE GUIDE:
EXPOSURES, TESTING, AND PREVENTION

Brucellosis Reference Guide: Exposures, Testing, and Prevention
CONTACT INFORMATION
Bacterial Special Pathogens Branch (BSPB)
Division of High-Consequence Pathogens and Pathology
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
1600 Clifton Rd MS C–09, Atlanta, GA 30329-4027
(404) 639-1711, [email protected]
Division of Select Agents and Toxins (DSAT)
Centers for Disease Control and Prevention
1600 Clifton Rd MS A–46, Atlanta, GA 30329-4027
Fax: (404) 718-2096, [email protected]
http://www.selectagents.gov/
Emergency Operations Center (EOC)
Office of Public Health Preparedness and Response
Centers for Disease Control and Prevention
(770) 488-7100
Laboratory Preparedness and Response Branch (LRN)
Division of Preparedness and Emerging Infection
National Center for Emerging, Zoonotic and Infectious Disease
Centers for Disease Control and Prevention
1600 Clifton Rd MS C–18, Atlanta, GA 30329-4027
Updated February 2017

Brucellosis Reference Guide: Exposures, Testing, and Prevention
TABLE OF CONTENTS
Contact Information ............................................................................................................ inside cover
Description ..........................................................................................................................................1
Clinical Description ..........................................................................................................................1
Case Classification
1, 2
........................................................................................................................ 1
Human Pathogens and Select Agent Reporting .................................................................................... 2
Select Agent Designation .................................................................................................................. 2
Laboratory Response Network (LRN)
6
.............................................................................................3
Reportable and Nationally Notifiable Disease Classification and Requirements
7
................................3
Case Report Form ............................................................................................................................ 4
Diagnostic Testing ...............................................................................................................................4
CDC/CSTE Laboratory Criteria for Diagnosis
1
................................................................................... 4
Testing performed at CDC ................................................................................................................5
Diagnostic Difficulties ......................................................................................................................6
Treatment
13, 14
......................................................................................................................................7
Laboratory, Surgical, and Clinical Exposures ........................................................................................8
Laboratory Exposures
4, 15
..................................................................................................................8
Clinical Exposure ........................................................................................................................... 12
Surgical Exposure
22, 23
..................................................................................................................... 12
Veterinary Exposures ......................................................................................................................... 14
Vaccine Exposure ........................................................................................................................... 14
Clinical Exposure ........................................................................................................................... 15
Marine Mammal Exposure
28, 29
........................................................................................................ 15
Foodborne Exposure ......................................................................................................................... 16
Recreational Exposure ....................................................................................................................... 17
Feral Swine Hunting ....................................................................................................................... 17
Brucellosis in Pregnant Women ......................................................................................................... 17
Person-to-Person Transmission ......................................................................................................... 18
Neonatal Brucellosis
36–40
................................................................................................................. 18
Sexual Transmission
41-48
.................................................................................................................. 19
Organ Donations and Blood Transfusions ....................................................................................... 19
Prevention ......................................................................................................................................... 19
Occupational Exposures ................................................................................................................. 19
Recreational Exposures (Hunter Safety) ..........................................................................................20
Travel to Endemic Areas ................................................................................................................. 20

Brucellosis Reference Guide: Exposures, Testing, and Prevention
References ......................................................................................................................................... 21
Additional Sources of Brucellosis Information: ...................................................................................24
Appendix 1: Specimen Submission .....................................................................................................26
Submission of Serum for Brucella Serology ....................................................................................... 26
Submission of Brucella Isolate(s) ...................................................................................................... 27
Appendix 2: Post-Exposure Monitoring .............................................................................................28
Follow-up of Brucella occupational exposure .................................................................................... 28
Symptom Monitoring ..................................................................................................................... 28
Appendix 3: Interim Marine Mammal Biosafety Guidelines ................................................................ 32

1
Brucellosis Reference Guide: Exposures, Testing, and Prevention
DESCRIPTION
Clinical Description
Council of State and Territorial Epidemiologists (CSTE)
1
2010 Case Definition
An illness characterized by acute or insidious onset of fever and one or more of the following: night sweats,
arthralgia, headache, fatigue, anorexia, myalgia, weight loss, arthritis/spondylitis, meningitis, or focal organ
involvement (endocarditis, orchitis/epididymitis, hepatomegaly, splenomegaly).
Incubation Period
●
Highly variable (5 days–6 months)
●
Average onset 2–4 weeks
Symptoms/Signs
●
Acute
Non-specific: Fever, chills, sweats, headache, myalgia, arthralgia, anorexia, fatigue, weight loss
Sub-clinical infections are common
Lymphadenopathy (10–20%), splenomegaly (20–30%)
Chronic
Recurrent fever
Arthritis and spondylitis
Possible focal organ involvement (as indicated in the case definition)
Case Classication
1, 2
Probable—A clinically compatible illness with at least one of the following:
●
Epidemiologically linked to a confirmed human or animal brucellosis case
●
Presumptive laboratory evidence, but without definitive laboratory evidence, of Brucella infection
Confirmed—A clinically compatible illness with definitive laboratory evidence of Brucella infection
Please refer to the CSTE Laboratory Criteria for Diagnosis section for specifications regarding “presumptive”
and “definitive” laboratory evidence of a Brucella infection.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
2
HUMAN PATHOGENS AND SELECT AGENT REPORTING
Select Agent Designation
Select agents and toxins are a subset of biological agents and toxins that may pose a severe threat to public
health.
3
Brucella species are easily aerosolized and have a low infectious dose, cited at levels between 10 and
100 microorganisms. These organisms also have a prolonged incubation period with the potential to induce
a broad range of clinical manifestations, and therefore generate challenges for prompt diagnosis. The above
factors have contributed to a select agent designation for B. suis, B. melitensis, and B. abortus.
4, 5
Clinical or diagnostic laboratories and other entities that have identified B. suis, B. melitensis, or B. abortus
are required to immediately (within 24 hours) notify the Division of Select Agents and Toxins (DSAT) at CDC
(fax: 404-718-2096; email: [email protected]).
Facilities that use or transfer B. suis, B. melitensis, or B. abortus must immediately (within 24 hours) notify DSAT
via phone, fax, or e-mail if they detect any theft, loss, or release of these select agents. The initial report should
include as much information as possible about the incident, including the type of incident, date and time,
agent and quantity, and a summary of the events (location of the incident, number of individuals potentially
exposed, actions taken to respond, etc.). Additionally, appropriate local, state, or federal law enforcement
agencies should be contacted of a theft or loss, and appropriate local, state, and federal health agencies
notified of a release.
3
Forms for Reporting to CDC’s Division of Select Agents and Toxins (DSAT)
Form 4, Report of the Identification of a Select Agent or Toxin: Clinical or APHIS/CDC Form 4A should be
signed and submitted after a facility has identified B. suis, B. melitensis, or B. abortus contained in a clinical/
diagnostic specimen. Entities must submit Form 4 to DSAT within 7 calendar days of identification. For
assistance with the completion of this form, please contact CDC’s Division of Select Agents and Toxins via
email at [email protected].
Form 3, Report of Theft, Loss, or Release of Select Agents and Toxins: The APHIS/CDC Form 3 should be
submitted by facilities reporting a theft, loss, or release (laboratory exposure or release of an agent outside
of the primary barriers of the biocontainment area) of B. suis, B. melitensis, or B. abortus within 7 calendar days
of the event. For reporting of a theft or loss, complete sections 1 and 2 of Form 3; for reporting a release,
complete sections 1, 2, and 3. With questions regarding the Form 3, please send an e-mail to [email protected].
Exclusions from Select Agent Reporting
Select agents in their naturally occurring environment are not subject to regulation and may include animals
that are naturally infected with a select agent or toxin (e.g., milk samples that contain B. abortus). However, a
select agent or toxin that has been intentionally introduced (e.g., animal experimentally infected with B. suis,
B. melitensis, or B. abortus), or otherwise extracted from its natural source (e.g., blood from a culture bottle is
plated onto agar and grows B. suis) is subject to select agent regulation.
3
Attenuated vaccine strains of B. abortus Strain 19 live vaccine and B. abortus Strain RB51 are excluded from
select agent reporting requirements, unless there is any reintroduction of factor(s) associated with virulence.
3
Visit the Select Agents and Toxins Exclusions site for a comprehensive list of attenuated Brucella strain
exclusions. Please refer to the APHIS/CDC Form 5, Request for Exemption of Select Agents and Toxins for
an Investigational Product, to request an exemption from the select agent regulations.

3
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Laboratory Response Network (LRN)
6
The LRN is a national network of local, state, federal, military, and international public health, food testing,
veterinary diagnostic, and environmental testing laboratories that provides laboratory infrastructure and
capacity to respond to biological and chemical public health emergencies.
Upon obtaining high confidence presumptive or confirmatory Brucella spp. results, LRN laboratories are
required to follow notification and messaging procedures.
Notification: Within 2 hours of obtaining high-confidence presumptive or confirmatory result, a LRN
Laboratory Director or a designee must notify:
●
their State Public Health Laboratory Director,
●
the State Epidemiologist,
●
the Health Officer for the State Public Health Department,
●
the CDC Emergency Operations Center (EOC), and
●
the FBI Weapons of Mass Destruction (WMD) POC.
Messaging: For emergency and non-emergency situations, LRN laboratories will submit data for all samples,
including positive and negative results related to the event within 12 hours of obtaining each result.
Please refer to Table 1 below for information regarding personnel who should be contacted, along with a
timeline for notification and messaging communication.
Reportable and Nationally Notiable Disease Classication and Requirements
7
Brucellosis is a reportable disease in all 57 states and territories; it is mandatory that disease cases be reported
to state and territorial jurisdictions when identified by a health provider, hospital, or laboratory. Reporting
requirements vary by jurisdiction.
Brucellosis is also a nationally notifiable condition. Notification of brucellosis cases (without direct personal
identifiers) to CDC by state and territorial jurisdictions is voluntary for nationwide aggregation and monitoring
of disease data. The case definition for confirmed and probable brucellosis can be found on page 3 under the
Case Classification section.
Immediate, Urgent Notification Status: For multiple confirmed and probable cases, temporally or spatially
clustered, notify EOC within 24 hours of a case meeting the notification criteria, followed by submission of
electronic case notification in the next regularly scheduled electronic transmission.
Standard: For confirmed and probable cases that are not temporally or spatially clustered, submit electronic
case notification within the next reporting cycle.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
4
Table 1: Reporting Known Brucella spp. Human Pathogens
Brucella species
Select Agent
Designation
Reporting Notification Status
DSAT
LRN
Nationally
Notifiable
Condition
Identification
(Form 4)
Exposure
(Form 3)
Brucella melitensis
Select Agent
24 hours
For registered
entities
7 days
For
non-registered
entities
7 days 2 hours 24 hours or 7 days
Brucella suis 7 days 2 hours 24 hours or 7 days
Brucella abortus 7 days 2 hours 24 hours or 7 days
Brucella canis Not a Select Agent N/A N/A 2 hours 24 hours or 7 days
Brucella ceti, pinnipedialis Not a Select Agent N/A N/A 2 hours 24 hours or 7 days
Case Report Form
Health departments and providers are strongly encouraged to use the approved case report form to report
brucellosis cases to the Bacterial Special Pathogens Branch. This mechanism will ensure improved collection
of standardized data needed to assess risk factors and trends associated with brucellosis, so that targeted
preventive strategies can be implemented. Patient identifiers such as full name, address, phone number,
hospital name, and medical record number should not be included in forms sent to CDC. Instructions for
completion and submission are included in pages 1 and 2 of the form.
DIAGNOSTIC TESTING
CDC/CSTE Laboratory Criteria for Diagnosis
1
Definitive
●
Culture and identification of Brucella spp. from clinical specimens
●
Evidence of a four-fold or greater rise in Brucella antibody titer between acute and convalescent phase
serum specimens obtained greater than or equal to 2 weeks apart
Presumptive
●
Brucella total antibody titer of greater than or equal to 1:160 by standard tube agglutination test (SAT)
or Brucella microagglutination test (BMAT) in one or more serum specimens obtained after onset of
symptoms
●
Detection of Brucella DNA in a clinical specimen by PCR assay
NOTE: Evidence of Brucella antibodies by nonagglutination-based tests
DOES NOT meet the current CDC/CSTE case definition for a
Presumptive Diagnosis of brucellosis.
However, ANY quantitative test can be used for confirmation if
there is a four-fold or greater rise in Brucella antibody titer.

5
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Testing Performed at CDC
The Zoonotic and Select Agent Laboratory (ZSAL) at CDC performs CLIA-approved Brucella spp. diagnostic
testing on human and animal samples.
Table 2: Diagnostic Testing Provided by ZSAL
Test
Samples
accepted
Pros Cons
Submission
Instructions
Culture
Tissue, whole
blood, sera,
plasma
Gold standard; allows
for genotyping-
molecular epidemiology
Requires BSL-3
See Appendix 1:
Submission of
Brucella Isolates
LRN PCR
(for suspect
BT and
response use)
Environmental
samples, swabs,
powders, whole
blood, sera, tissue
Rapid detection; can
be used on isolates and
clinical specimens
Requires technical expertise
to perform assay; reagents
and equipment can be
costly; optimal specimen
type not clear
See Appendix 1:
Submission of
Brucella Isolates
MAT
(serology)
Not available for
B. canis or RB51
Sera
Cheap, assay of
choice in acute non-
complicated cases; only
equipment needed is
reading apparatus
May not diagnose chronic
or complicated cases;
subjective
See Appendix 1:
Submission of Serum
for Brucella Serology
Results and Notification
●
BMAT results take 2 to 3 weeks, depending on when your sample was received at CDC’s Zoonotic and
Select Agent Laboratory (ZSAL), which is part of the Bacterial Special Pathogens Branch (BSPB). Our lab
will generally test your sample within 1 week; however, it can take longer to report results. Results will be
sent to your State Laboratory.
●
After checking with your State Laboratory, you can contact ZSAL or the BSPB epidemiology team if results
are not received within 2 to 3 weeks.
●
PCR results from primary specimens can be obtained within 24 hours.
Testing Performed Elsewhere
●
CDC does not provide medical consultation on individual patients, and cannot comment on results from
laboratory assays that we have not performed.
●
We recommend that you consult both with an infectious disease specialist assigned to the patient and the
medical director for the diagnostic laboratory that ran the tests for interpretation of results, as they have
the available parameters for the specific assay used.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
6
Diagnostic Difculties
While culture is the gold standard, Brucella spp. can be fastidious, slow growers. Culture from primary
specimens may require up to 21 days of incubation. Bone marrow culture is more sensitive than blood;
however, the invasiveness of the procedure should be considered. Persons with chronic infections are less likely
to be culture-positive.
Agglutination is a confirmatory serological test to diagnose brucellosis. The standard tube agglutination test
(SAT) is the reference method, of which BMAT is a modified format.
●
Brucella-specific agglutination tests involve direct agglutination of bacterial antigens by specific antibodies.
Agglutination tests detect antibodies of IgM, IgG, and IgA classes.
●
IgM antibodies are predominant in acute infection but decline within weeks. Relapses are accompanied by
transient elevations of IgG and IgA antibodies but not IgM.
Stage of Brucellosis
nonagglutinating IgA
nonagglutinating IgG
IgG
IgM
Acute exacerbation
Acute
(up to 3 mo.)
IgM
IgG
IgA
Subacute
(3 mo. - 1 yr)
Chronic
(1 yr. onward)
Antibody
Levels
Positive
Blood
Cultures
Figure 1: Antibody Responses in Untreated Brucellosis.
8
IgM detection sensitivities using other EIA formats have been reported between 67% to 100% with limited
specificity data.
9, 10
Such tests are qualitative, making them difficult to interpret in a clinical setting, and might
have different performance characteristics and utility when used in areas with low disease prevalence, such as
the United States. Results of EIA tests must be confirmed by a quantitative reference method such as BMAT.
BMAT Testing Drawbacks
Cross-reactions and false-positive test results can occur in Brucella antibody tests, mainly with IgM.10
The primary immunodeterminant and virulence factor for Brucella species is smooth lipopolysaccharide (S-LPS)
on the outer cell membrane, which is antigenically similar to the lipopolysaccharide of other gram-negative
rods. False-positive Brucella test results can be caused by cross-reactivity of antibodies to Escherichia coli O157,
Francisella tularensis, Moraxella phenylpyruvica, Yersinia enterocolitica, certain Salmonella serotypes, and from persons
vaccinated against Vibrio cholerae.
BMAT tends to perform better for diagnosing acute cases rather than chronic cases.
11
Additionally, BMAT is
not as useful in detecting chronic brucellosis cases or neurobrucellosis. In a suspected chronic case, an IgG
ELISA would be more informative.
12

7
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Treatment
13, 14
The table below provides a summary of the Red Book treatment recommendations, as well as several
recommended treatment documents. Information about post-exposure prophylaxis is provided below in the
section titled “Laboratory, Surgical, and Clinical Exposures.”
Table 3: Brucellosis Treatment Options
Subject Summary
Adults,
Children > 8 years
Combination therapy to decrease the incidence of relapse:
● Oral doxycycline (2–4 mg/kg per day, maximum 200 mg/day, in 2 divided doses) or oral
tetracycline (30–40 mg/kg per day, maximum 2 g/day, in 4 divided doses) -and-
● Rifampin (15–20 mg/kg per day, maximum 600–900 mg/day, in 1 or 2 divided doses).
● Recommended for a minimum of 6 weeks.
Combination therapy with trimethoprim-sulfamethoxazole (TMP-SMZ) can be used if
tetracyclines are contraindicated.
Children < 8 years
● Oral TMP-SMZ (trimethoprim, 10 mg/kg per day, maximum 480 mg/day; and sulfamethoxazole,
50 mg/kg per day, maximum 2.4 g/day) divided in 2 doses for 4 to 6 weeks.
Combination therapy: consider adding rifampin. Consult physician for dosing or if
rifampin is contraindicated. Tetracyclines (such as doxycycline) should be avoided in
children less than 8 years of age.
Pregnancy
Tetracyclines are contraindicated for pregnant patients. Consult obstetrician regarding
specific antimicrobial therapy instructions.
Complicated Cases
(endocarditis,
meningitis,
osteomyelitis, etc.)
● Streptomycin* or gentamicin for the first 14 days of therapy in addition to a
tetracycline for 6 weeks (or TMP-SMZ if tetracyclines are contraindicated).
● Rifampin can be used in combination with this regimen to decrease the rate of relapse.
● For life-threatening complications, such as meningitis or endocarditis, duration of
therapy often is extended for 4 to 6 months.
● Case-fatality rate is < 1%.
● Surgical intervention should be considered in patients with complications such as
deep tissue abscesses.
*May not be readily available in the U.S.
References
for Treatment
Recommendations
● Ariza J et al. 2007. Perspectives for the Treatment of Brucellosis in the 21st Century:
The Ioannina Recommendations. PLoS Med. 4(12): e317. http://www.plosmedicine.
org/article/info:doi/10.1371/journal.pmed.0040317
● Al-Tawfiq JA. 2008. Therapeutic options for human brucellosis. Expert Rev Anti Infect
Ther. 6(1): 109-120. http://www.ncbi.nlm.nih.gov/pubmed/18251668
● Solera J. 2010. Update on brucellosis: therapeutic challenges. Intl J Antimicrob Agent.
36S, S18–S20. http://www.ncbi.nlm.nih.gov/pubmed/20692127
Note: The B. abortus strain used in the RB51 vaccine was derived by selection in rifampin-enriched media and is resistant to rifampin in vitro.
This strain is also resistant to penicillin. If infection is due to this vaccine strain, treatment should be determined accordingly (example:
doxycycline and TMP/SMX in place of rifampin). Specifics on the regimen and dose should be established in consultation with the person’s
health care provider in case of contraindications to the aforementioned.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
8
LABORATORY, SURGICAL, AND CLINICAL EXPOSURES
Laboratory Exposures
4, 15
Once a potential exposure is recognized, the first task is to determine the activities performed that may have
led to the exposure. Then identify:
1. who was in the laboratory during the suspected time(s) of exposure
2. where they were in relation to the exposure
3. what they did with the isolates
The identified individuals should be assessed for exposure risk using the descriptions in Table 4.
Table 4. Laboratory Risk Assessment and Post-Exposure Prophylaxis (PEP): Minimal (but not zero) Risk
Specimen
handling
Exposure scenario PEP Follow-up/monitoring
Routine clinical
specimen (e.g.,
blood, serum,
cerebrospinal
fluid)
Person who manipulates a routine
clinical specimen (e.g., blood, serum,
cerebrospinal fluid) in a certified Class
II biosafety cabinet, with appropriate
personal protective equipment (i.e.,
gloves, gown, eye protection).
None
N/A
May consider symptom watch for
following scenarios:
● Person who manipulates a routine
clinical specimen (e.g., blood,
serum, cerebrospinal fluid) on
an open bench with or without
appropriate personal protective
equipment (i.e., gloves, gown,
eye protection), or in a certified
Class II biosafety cabinet without
appropriate personal protective
equipment.
● Person present in the lab while
someone manipulates a routine
clinical specimen (e.g., blood,
serum, cerebrospinal fluid) on
an open bench, resulting in
occurrence of aerosol-generating
events (e.g., centrifuging without
sealed carriers, vortexing,
sonicating, spillage/splashes).
Person present in the lab while someone
manipulates a routine clinical specimen
(e.g., blood, serum, cerebrospinal fluid)
in a certified Class II biosafety cabinet,
or on an open bench where manipulation
did not involve occurrence of aerosol-
generating events (e.g., centrifuging
without sealed carriers, vortexing,
sonicating, spillage/splashes).
Enriched material
(e.g., a Brucella
isolate, positive
blood bottle)
or reproductive
clinical specimen
(e.g., amniotic
fluid, placental
products)
Person who manipulates enriched material
(e.g., a Brucella isolate, positive blood
bottle) or reproductive clinical specimen
(e.g., amniotic fluid, placental products)
in a certified Class II biosafety cabinet,
with appropriate personal protective
equipment (i.e., gloves, gown, eye
protection).
Person present in the lab while someone
manipulates enriched material (e.g., a
Brucella isolate, positive blood bottle)
or reproductive clinical specimen (e.g.,
amniotic fluid, placental products) in
a certified Class II biosafety cabinet.

9
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Table 4. Laboratory Risk Assessment and Post-Exposure Prophylaxis (PEP): Low Risk
Specimen
handling
Exposure scenario PEP
Follow-up/
monitoring
Enriched material
(e.g., a Brucella
isolate, positive
blood bottle)
or reproductive
clinical specimen
(e.g., amniotic
fluid, placental
products)
Person present in the lab at a distance
of greater than 5 feet from someone
manipulating enriched material (e.g., a
Brucella isolate, positive blood bottle)
or reproductive clinical specimen (e.g.,
amniotic fluid, placental products),
on an open bench, with no occurrence
of aerosol-generating events (e.g.,
centrifuging without sealed carriers,
vortexing, sonicating, spillage/splashes).
May consider if
immunocompromised
or pregnant.
Discuss with health
care provider (HCP).
Note: RB51 is
resistant to rifampin
in vitro, and therefore
this drug should not
be used for PEP or
treatment courses.
Regular symptom
watch (e.g., weekly)
and daily self-fever
checks through
24 weeks post-
exposure, after last
known exposure.
Sequential
serological
monitoring at 0
(baseline), 6, 12, 18,
and 24 weeks post-
exposure, after last
known exposure.
Note: no serological
monitoring
currently available
for RB51 and B.
canis exposures in
humans.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
10
Table 4. Laboratory Risk Assessment and Post-Exposure Prophylaxis (PEP): High Risk
Specimen
handling
Exposure scenario PEP
Follow-up/
monitoring
Roune clinical
specimen (e.g.,
blood, serum,
cerebrospinal
uid)
Person who manipulates a roune clinical
specimen (e.g., blood, serum, cerebrospinal
uid), resulng in contact with broken skin or
mucous membranes, regardless of working
in a cered Class II biosafety cabinet, with
or without appropriate personal protecve
equipment (i.e., gloves, gown, eye protecon).
Doxycycline 100mg
twice daily, and
rifampin 600 mg once
daily, for three weeks.
For paents with
contraindicaons
to doxycycline or
rifampin: TMP-
SMZ, in addion to
another appropriate
anmicrobial, should
be considered.
Two anmicrobials
eecve against
Brucella should be
given.
Pregnant women
should consult their
obstetrician.
Note: RB51 is resistant
to rifampin in vitro,
and therefore this
drug should not
be used for PEP or
treatment courses.
Regular symptom
watch (e.g.,
weekly) and
daily self-fever
checks through
24 weeks post-
exposure, aer
last known
exposure.
Sequenal
serological
monitoring at 0
(baseline), 6, 12,
18, and 24 weeks
post-exposure,
aer last known
exposure.
Note: no
serological
monitoring
currently
available for
RB51 and B. canis
exposures in
humans.
Enriched
material (e.g., a
Brucella isolate,
posive blood
bole) or
reproducve
clinical specimen
(e.g., amnioc
uid, placental
products)
Person who manipulates (or is ≤ 5 feet from
someone manipulang) enriched material
(e.g., a Brucella isolate, posive blood bole)
or reproducve clinical specimen (e.g.,
amnioc uid, placental products), outside of
a cered Class II biosafety cabinet.
Person who manipulates enriched material
(e.g., a Brucella isolate, posive blood bole)
or reproducve clinical specimen (e.g.,
amnioc uid, placental products), within a
cered Class II biosafety cabinet, without
appropriate personal protecve equipment
(i.e., gloves, gown, eye protecon).
All persons present during the occurrence of
aerosol-generang events (e.g., centrifuging
without sealed carriers, vortexing, sonicang,
spillage/splashes) with manipulaon of
enriched material (e.g., a Brucella isolate,
posive blood bole) or reproducve clinical
specimen (e.g., amnioc uid, placental
products) on an open bench.
Widespread aerosol generating procedures include, but are not limited to: centrifuging without sealed carriers,
vortexing, sonicating, or accidents resulting in spillage or splashes (i.e. breakage of tube containing specimen).
Other manipulations such as automated pipetting of a suspension containing the organism, grinding the
specimen, blending the specimen, shaking the specimen or procedures for suspension in liquid to produce
standard concentration for identification may require further investigation (i.e. inclusion of steps that could be
considered major aerosol generating activities).
Antimicrobial Post-Exposure Prophylaxis (PEP)
4, 15
Workers with high-risk exposures should begin antimicrobial post-exposure prophylaxis as soon as possible.
Prophylaxis can be initiated up to 24 weeks after exposure. PEP is generally not recommended for low-risk
exposures, though it may be considered on a case-by-case basis. PEP courses should include doxycycline
(100 mg) orally twice daily and rifampin (600 mg) once daily for a minimum of 21 days. Trimethoprim-
sulfamethoxazole (TMP-SMZ) or another antimicrobial agent effective against Brucella should be selected (for

11
Brucellosis Reference Guide: Exposures, Testing, and Prevention
at least 21 days) if doxycycline or rifampin are contraindicated. All PEP regimen and dosing decisions should
be made in consultation with the worker’s health care provider. If clinical symptoms develop at any point while
on PEP and brucellosis infection is confirmed by culture and isolation or serology, PEP is no longer appropriate
and treatment and monitoring is required.
Persons who are pregnant, less than 8 years old, or have contraindications to these antimicrobial agents,
should consult with their health care provider for alternative PEP. Suitable combinations of agents may be
selected from the treatment references listed previously.
●
Exposure to B. abortus RB51: Upon exposure to rifampin-resistant B. abortus RB51 vaccine, PEP should be
comprised of doxycycline in addition to another suitable antimicrobial (such as TMP-SMX) for 21 days.
16
Specifics on the regimen and dose should be established in consultation with the person’s health care
provider in case of contraindications to the aforementioned.
Symptom Surveillance
4
An occupational health provider should arrange for regular (at least weekly) monitoring for febrile illness or
symptoms consistent with brucellosis for all exposed workers. In addition, daily self-administered temperature
checks are recommended for 24 weeks post-exposure, from the last known date of exposure. Exposed persons
should be informed of common brucellosis symptoms, and are encouraged to seek immediate medical
treatment if illness develops within 6 months of the exposure, regardless of whether or not the patient has
already undergone PEP. It is important for workers to notify their health care provider of their recent Brucella
exposure so that receiving diagnostic laboratories may be notified and take precautions. Individuals who have
risk factors for relapse of brucellosis
17
may require a follow-up time that extends beyond 24 weeks.
●
B. canis and B. abortus RB51: Symptom monitoring should be emphasized following exposures to
Brucella canis and Brucella abortus RB51 vaccine due to the lack of serological tests available to identify
seroconversion.
Specific information regarding common symptoms of brucellosis and a symptom-monitoring table are
available in Appendix 2. These tools can be distributed to occupational health staff.
Serological Monitoring
4
All exposed workers should undergo quantitative serological testing in order to detect an immune response to
Brucella spp. Evidence suggests that seroconversion can occur shortly before symptoms appear, and therefore
may be an earlier indicator of infection. It is recommended that sera be drawn and submitted to the same
laboratory at 0 (baseline), 6, 12, 18, and 24 weeks following the exposure event.
CDC’s Zoonotic and Select Agent Laboratory (ZSAL) is able to perform serial serological monitoring at no
cost. If monitoring is conducted by other laboratories, it is recommended that an agglutination assay is used
to quantify seroconversion. Instructions for serology submission to ZSAL are available in Appendix 1.
●
B. canis and B. abortus RB51: Serological testing is currently not available for Brucella canis and Brucella
abortus RB51 vaccine. Serological monitoring following exposure to these strains is not recommended,
except to collect a baseline serum sample in order to rule out infection with other Brucella spp.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
12
Clinical Exposure
Universal precautions and personal
protective equipment (PPE) are essential
when working with body fluids or tissues
from a brucellosis patient. When standard
precautions are followed, most clinical
procedures are considered to be low-risk
activities. Higher-risk activities may include
handling of tissues with potentially high
concentrations of Brucella organisms (e.g.,
placental tissues), direct contact with
infected blood and body fluids through
breaks in the skin, or mucosal
exposure to aerosolized Brucella organisms
after an aerosol-generating procedure.
Aerosol-Generating Procedures: Aerosols
are defined as particulates (diameter < 10
µm) suspended in the air. Aerosol-generating
procedures are those that produce aerosols
as a result of mechanical disturbance of the blood or another body fluid
18
. Aerosol-generating procedures may
include, but are not limited to, cardiopulmonary resuscitation, disturbance of fluids from an abscess, the use
of saws or other electrical devices, and high-pressure irrigation. Additional information on the utilization of
electrical and irrigation devices can be found in the Surgical Exposure section below.
To the best of our knowledge, seven cases of occupationally acquired brucellosis have been reported in
the English literature among health care workers since 1990, including four infections acquired during
obstetrical delivery, and three infections through the provision of medical care to brucellosis patients. In
each case, it is likely that the health care workers were exposed through the high-risk routes of transmission
previously listed (handling of placental tissues, direct contact with infected blood/tissues, and mucosal
exposure to aerosolized Brucella).
19-21
Surgical Exposure
22, 23
In the event of a Brucella exposure during a surgical procedure, the potential risk of exposure should be
evaluated for all personnel who pass through the surgical unit. Assessments should be based on adherence
to PPE requirements, types of surgical devices utilized, risk of aerosolization, and duration of the surgical
procedure. The following paragraph, along with Table 5, may be used as a resource for risk assessment.
Risk Assessment: High-risk exposures within surgical settings have previously been defined as presence
within an operating room during aerosol-generating event, and cleaning the operating room after an aerosol-
generating procedure. Aerosol-generating procedures may include, but are not limited to, the use of saws
or other electrical devices, cardiopulmonary resuscitation, disturbance of fluids from an abscess, and high-
pressure irrigation. Risk of aerosolization subsequent to irrigation should be assessed based upon the water
pressure from the irrigation tool used. High-pressure washes and pulsed lavages are generally considered to be
high-pressure irrigation, and the use of such devices should be treated as an aerosol-generating event. While
hand bulbs are typically considered to be a low-pressure irrigation device, additional factors, like surgical
technique, should be considered before ruling out this mechanism as an aerosol-generating procedure.

13
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Clinical Exposure
Universal precautions and personal
protective equipment (PPE) are essential
when working with body fluids or tissues
from a brucellosis patient. When standard
precautions are followed, most clinical
procedures are considered to be low-risk
activities. Higher-risk activities may include
handling of tissues with potentially high
concentrations of Brucella organisms (e.g.,
placental tissues), direct contact with
infected blood and body fluids through
breaks in the skin, or mucosal
exposure to aerosolized Brucella organisms
after an aerosol-generating procedure.
Aerosol-Generating Procedures: Aerosols
are defined as particulates (diameter < 10
µm) suspended in the air. Aerosol-generating
procedures are those that produce aerosols
as a result of mechanical disturbance of the blood or another body fluid
18
. Aerosol-generating procedures may
include, but are not limited to, cardiopulmonary resuscitation, disturbance of fluids from an abscess, the use
of saws or other electrical devices, and high-pressure irrigation. Additional information on the utilization of
electrical and irrigation devices can be found in the Surgical Exposure section below.
To the best of our knowledge, seven cases of occupationally acquired brucellosis have been reported in
the English literature among health care workers since 1990, including four infections acquired during
obstetrical delivery, and three infections through the provision of medical care to brucellosis patients. In
each case, it is likely that the health care workers were exposed through the high-risk routes of transmission
previously listed (handling of placental tissues, direct contact with infected blood/tissues, and mucosal
exposure to aerosolized Brucella).
19-21
Surgical Exposure
22, 23
In the event of a Brucella exposure during a surgical procedure, the potential risk of exposure should be
evaluated for all personnel who pass through the surgical unit. Assessments should be based on adherence
to PPE requirements, types of surgical devices utilized, risk of aerosolization, and duration of the surgical
procedure. The following paragraph, along with Table 5, may be used as a resource for risk assessment.
Risk Assessment: High-risk exposures within surgical settings have previously been defined as presence
within an operating room during aerosol-generating event, and cleaning the operating room after an aerosol-
generating procedure. Aerosol-generating procedures may include, but are not limited to, the use of saws
or other electrical devices, cardiopulmonary resuscitation, disturbance of fluids from an abscess, and high-
pressure irrigation. Risk of aerosolization subsequent to irrigation should be assessed based upon the water
pressure from the irrigation tool used. High-pressure washes and pulsed lavages are generally considered to be
high-pressure irrigation, and the use of such devices should be treated as an aerosol-generating event. While
hand bulbs are typically considered to be a low-pressure irrigation device, additional factors, like surgical
technique, should be considered before ruling out this mechanism as an aerosol-generating procedure.
Pre-Operative Recommendations for Surgery on a Brucellosis Patient:
●
The patient should be started on antibiotic therapy to decrease the bacterial load of the surrounding
tissues. Table 3 can be utilized for treatment guidance.
●
Precautions to be taken by medical staff prior to and during the operation:
Minimize aerosol-generating procedures during the surgical procedure
Only essential personnel should be present in the operating room during the procedure
All staff members present in the operating room should wear appropriate PPE, including:
❍
Gloves, masks, and eyewear
❍
Respiratory protection (e.g., N95) if there is potential for aerosol-generating procedures
Post-Operative Recommendations for Surgery on a Brucellosis Patient:
●
Evaluation of staff after potential exposure to Brucella organisms should include:
A review of appropriate PPE and possible breaches in PPE protocol during the surgical
procedure, including:
❍
Symptom and serological monitoring (as applicable) for all personnel for whom a breach of PPE
is identified.
❍
Consideration of PEP for all personnel who were present during or after a potential aerosol-
generating procedure was done.
●
Serological monitoring (as applicable) and PEP consideration for staff who are pregnant or
immunocompromised.
Workers are advised to seek medical consultation with their health care provider.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
14
VETERINARY EXPOSURES
Vaccine Exposure
Accidental exposures to live, attenuated vaccine strains of
Brucella spp. in veterinarians have been reported via needle stick
injury, as well as through spray exposure to the conjunctiva and
open wounds. Personnel administering RB51, S19, and Rev-1
vaccinations should wear proper PPE, including gloves and eye
protection. Proper animal restraint should be used to minimize
needle sticks or conjunctival splashes.
Brucella abortus RB51 Vaccine
16, 24
The Brucella abortus RB51 vaccine is currently the only vaccine
used in the United States for prevention of brucellosis in cattle
herds. Although RB51 was developed to be less pathogenic
and abortifacient than the S19 strain in animals, it does
retain pathogenicity for humans. Local adverse events have
been reported less than 24 hours after exposure, and systemic
reactions may begin 1 to 15 days subsequent to exposure.
●
Risk Assessment: Vaccine exposures typically occur
through direct contact; therefore, all individuals exposed to
RB51 should be considered as having a high-risk exposure.
●
Symptom Monitoring: Symptom monitoring should
be emphasized following exposures to RB51 vaccine
because of the lack of serological tests available to
identify seroconversion. The symptom monitoring table in
Appendix 2 can be given to exposed individuals.
●
Antimicrobial Post-Exposure Prophylaxis (PEP): Antibiotic post-exposure prophylaxis has been
recommended for individuals accidentally exposed to B. abortus RB51 vaccine. Refer to Table 4 for PEP
guidance. Because RB51 was derived by selection in rifampin-enriched media and is resistant to rifampin
in vitro, rifampin should not be used for PEP. The strain is also resistant to penicillin.
●
Serological Monitoring: The RB51 vaccine is a modified live culture vaccine and there are currently no
serological assays available to detect an antibody response to RB51.
Brucella abortus S19 Vaccine and Brucella melitensis Rev-1 Vaccine
25, 26
The B. abortus S19 and the B. melitensis Rev-1 animal brucellosis vaccines are available outside the U.S. and
have been known to cause systemic disease in humans. With the scope of human travel and animal trading,
potential cases in the U.S. may arise in persons exposed to the vaccine or to animals previously vaccinated.
●
Risk Assessment: Vaccine exposures typically occur through direct contact; therefore, all individuals
exposed to S19 or Rev-1 strains should be considered as having a high-risk exposure.
●
Symptom Monitoring: Guidelines for symptom monitoring can be found in Appendix 2.
●
Antimicrobial Post-Exposure Prophylaxis (PEP): CDC recommends a concomitant prophylaxis regimen of
doxycycline and rifampin for three weeks following exposure to the S19 and Rev-1 vaccine strains. Refer to
Table 4 for PEP guidance. Rev-1 is resistant to streptomycin, and therefore this drug should not be used for
PEP or treatment courses.
25

15
Brucellosis Reference Guide: Exposures, Testing, and Prevention
VETERINARY EXPOSURES
Vaccine Exposure
Accidental exposures to live, attenuated vaccine strains of
Brucella spp. in veterinarians have been reported via needle stick
injury, as well as through spray exposure to the conjunctiva and
open wounds. Personnel administering RB51, S19, and Rev-1
vaccinations should wear proper PPE, including gloves and eye
protection. Proper animal restraint should be used to minimize
needle sticks or conjunctival splashes.
Brucella abortus RB51 Vaccine
16, 24
The Brucella abortus RB51 vaccine is currently the only vaccine
used in the United States for prevention of brucellosis in cattle
herds. Although RB51 was developed to be less pathogenic
and abortifacient than the S19 strain in animals, it does
retain pathogenicity for humans. Local adverse events have
been reported less than 24 hours after exposure, and systemic
reactions may begin 1 to 15 days subsequent to exposure.
●
Risk Assessment: Vaccine exposures typically occur
through direct contact; therefore, all individuals exposed to
RB51 should be considered as having a high-risk exposure.
●
Symptom Monitoring: Symptom monitoring should
be emphasized following exposures to RB51 vaccine
because of the lack of serological tests available to
identify seroconversion. The symptom monitoring table in
Appendix 2 can be given to exposed individuals.
●
Antimicrobial Post-Exposure Prophylaxis (PEP): Antibiotic post-exposure prophylaxis has been
recommended for individuals accidentally exposed to B. abortus RB51 vaccine. Refer to Table 4 for PEP
guidance. Because RB51 was derived by selection in rifampin-enriched media and is resistant to rifampin
in vitro, rifampin should not be used for PEP. The strain is also resistant to penicillin.
●
Serological Monitoring: The RB51 vaccine is a modified live culture vaccine and there are currently no
serological assays available to detect an antibody response to RB51.
Brucella abortus S19 Vaccine and Brucella melitensis Rev-1 Vaccine
25, 26
The B. abortus S19 and the B. melitensis Rev-1 animal brucellosis vaccines are available outside the U.S. and
have been known to cause systemic disease in humans. With the scope of human travel and animal trading,
potential cases in the U.S. may arise in persons exposed to the vaccine or to animals previously vaccinated.
●
Risk Assessment: Vaccine exposures typically occur through direct contact; therefore, all individuals
exposed to S19 or Rev-1 strains should be considered as having a high-risk exposure.
●
Symptom Monitoring: Guidelines for symptom monitoring can be found in Appendix 2.
●
Antimicrobial Post-Exposure Prophylaxis (PEP): CDC recommends a concomitant prophylaxis regimen of
doxycycline and rifampin for three weeks following exposure to the S19 and Rev-1 vaccine strains. Refer to
Table 4 for PEP guidance. Rev-1 is resistant to streptomycin, and therefore this drug should not be used for
PEP or treatment courses.
25
●
Serological Monitoring: Serological monitoring is available for S19 and Rev-1 exposures. Quantitative
serological monitoring should be emphasized to detect a B. abortus S19 infection among veterinary workers,
as patients may present with mild clinical symptoms or as asymptomatic.
26
Clinical Exposure
Veterinarians and breeders have a higher risk of contracting brucellosis because of close direct contact with
infected animals, and in part because of inconsistency in the implementation of standard precautions in
veterinary practice.
Risk of exposure is greatest when veterinarians handle aborting animals or those undergoing parturition, though
high-risk activities may also include specimen draws during clinical examination, surgical procedures, or
disinfection and cleaning of contaminated environments. Inhalation of aerosolized Brucella organisms and
contamination of the conjunctiva or broken skin are common routes of exposure during the aforementioned
high-risk procedures.
Exposure to Brucella canis
While dogs can become infected with various Brucella spp., they serve
as the primary host for Brucella canis. B. canis is thought to be less
virulent than other strains of Brucella species and few human cases
have been documented, though this may be a result of difficulty in
diagnosis and underreporting.
27
●
Symptom Monitoring: Symptom monitoring should be
emphasized following exposures to dogs infected with brucellosis
because of the lack of serological tests available to identify
seroconversion. The symptom monitoring table in Appendix 2 can
be given to exposed individuals.
●
Serological Monitoring: While serological monitoring is not
available for B. canis exposures, it is recommended that baseline
serum is drawn for serological testing to rule out titers to other
Brucella spp., as veterinary personnel may be exposed to a variety
of species.
●
Antimicrobial Post-Exposure Prophylaxis (PEP): A prophylaxis
regimen should be considered for all personnel with high-risk
exposures. See Table 4 for PEP guidance.
Marine Mammal Exposure
28, 29
Multiple marine mammals that have been stranded in the Gulf of Mexico, Atlantic, and Pacific coasts since 2010
have had laboratory evidence of brucellosis infection. While marine-associated brucellosis in humans has not
been documented in the U.S., four human cases are known to have occurred worldwide. One individual was
exposed in a laboratory while handling samples from an infected dolphin, and three individuals became sick
after consuming raw fish or shellfish. Individuals who come in contact with marine mammals, particularly those
stranded or visibly ill, are potentially at risk for infection from B. ceti or B. pinnipedialis.
●
Risk Assessment: Higher-risk activities when working with infected marine mammals include aerosol-
generating procedures (use of saws) or cleaning of facilities with high-pressure equipment during and after
a necropsy. Failure to use PPE, including proper respiratory protection, during the aforementioned activities
places individuals at a greater risk for occupational exposure to Brucella spp. An excerpt of the Revised
Interim Marine Mammal Brucella Specific Biosafety Guidelines for the National Marine Mammal Stranding
Network is provided in Appendix 3, and may be used as a resource for post-exposure risk assessment.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
16
●
Symptom Monitoring: Persons experiencing signs and
symptoms through 24 weeks post-exposure to infected
marine mammals are encouraged to visit their local health
care provider as soon as possible for diagnosis, informing
their doctor that they may have been exposed to an
infectious zoonotic disease such as Brucella. The symptom-
monitoring table in Appendix 2 can be distributed to
individuals who have potentially been exposed.
●
Serological Monitoring: Serologic testing for B. ceti and
B. pinnipedialis can be done with the BMAT. Baseline sera
should be drawn for individuals at high risk as soon as
the exposure is recognized, and subsequently at 6-week
intervals through 24 weeks post-exposure following the
sequence for laboratory exposures.
●
Antimicrobial Post-Exposure Prophylaxis (PEP): Antimicrobial post-exposure prophylaxis
recommendations in the case of a marine mammal exposure are based on risk assessment for the exposed
person. See Table 4 for PEP guidance.
●
Reporting: Any human illness related to zoonotic disease exposure should be reported to the stranding
facility and the National Marine Fisheries Service (NMFS) Regional Office as soon as possible by
emailing the Regional Stranding Coordinators. The Regional Stranding Coordinators will notify the Marine
Mammal Health and Stranding Response Program (MMHSRP), who will contact the State Public Health
Veterinarian, the county and/or state department public health official and CDC.
FOODBORNE EXPOSURE
Approximately 70 to 75% of U.S. brucellosis cases
reported annually to CDC are due to B. melitensis and
B. abortus, and occur after individuals consume
unpasteurized dairy products from countries where
brucellosis remains endemic. Areas currently listed
as high-risk include: the Mediterranean Basin
(Portugal, Spain, Southern France, Italy, Greece,
Turkey, and North Africa), Mexico, South and
Central America, Eastern Europe, Asia, Africa,
the Caribbean, and the Middle East. Prevention
measures should focus on educating immigrants
and international travelers about the risks of
consuming unpasteurized dairy products from these
regions. Feral swine hunters who consume raw or
undercooked pork are also at risk for food-borne
exposure to brucellosis (via B. suis).
In cases of foodborne brucellosis, systemic symptoms are more commonly reported than gastrointestinal
complaints. A subset of patients experience nausea, vomiting, and abdominal discomfort, and rare cases of
ileitis, colitis, and spontaneous bacterial peritonitis have also been reported.
30
Individuals who become sick
with a febrile illness after consumption of unpasteurized dairy products or meat from feral swine should be
encouraged to submit samples of the food for culture and PCR to confirm the route of transmission.

17
Brucellosis Reference Guide: Exposures, Testing, and Prevention
●
Antimicrobial Post-Exposure Prophylaxis (PEP): Antimicrobial post-exposure prophylaxis
recommendations in the case of a marine mammal exposure are based on risk assessment for the exposed
person. See Table 4 for PEP guidance.
●
Reporting: Any human illness related to zoonotic disease exposure should be reported to the stranding
facility and the National Marine Fisheries Service (NMFS) Regional Office as soon as possible by
emailing the Regional Stranding Coordinators. The Regional Stranding Coordinators will notify the Marine
Mammal Health and Stranding Response Program (MMHSRP), who will contact the State Public Health
Veterinarian, the county and/or state department public health official and CDC.
FOODBORNE EXPOSURE
Approximately 70 to 75% of U.S. brucellosis cases
reported annually to CDC are due to B. melitensis and
B. abortus, and occur after individuals consume
unpasteurized dairy products from countries where
brucellosis remains endemic. Areas currently listed
as high-risk include: the Mediterranean Basin
(Portugal, Spain, Southern France, Italy, Greece,
Turkey, and North Africa), Mexico, South and
Central America, Eastern Europe, Asia, Africa,
the Caribbean, and the Middle East. Prevention
measures should focus on educating immigrants
and international travelers about the risks of
consuming unpasteurized dairy products from these
regions. Feral swine hunters who consume raw or
undercooked pork are also at risk for food-borne
exposure to brucellosis (via B. suis).
In cases of foodborne brucellosis, systemic symptoms are more commonly reported than gastrointestinal
complaints. A subset of patients experience nausea, vomiting, and abdominal discomfort, and rare cases of
ileitis, colitis, and spontaneous bacterial peritonitis have also been reported.
30
Individuals who become sick
with a febrile illness after consumption of unpasteurized dairy products or meat from feral swine should be
encouraged to submit samples of the food for culture and PCR to confirm the route of transmission.
RECREATIONAL EXPOSURE
Feral Swine Hunting
Approximately 25 to 30% of U.S. brucellosis cases
reported annually to CDC are due to B. suis and
almost all are diagnosed in feral swine hunters
(CDC, unpublished data). Feral swine have been
reported in at least 41 states, and serologic surveys
have detected endemic feral swine infection with
B. suis in 13 states (Arkansas, California, Florida,
Georgia, Hawaii, Louisiana, Mississippi, Missouri,
South Carolina, and Texas). Feral swine hunting is
allowed in most states with feral swine presence.
Out-of-state hunters often bring swine meat back
to their home states; therefore cases may occur even
in regions where B. suis is not endemic in feral swine
populations.
31
Efforts to prevent B. suis infection should focus on education of hunters and partnerships between state
and local public health, wildlife and agricultural agencies, as well as sportsmen’s associations. CDC feral
swine hunter brochures are available for public dissemination and can be found in the Additional Sources of
Brucellosis Information section.
B. suis in Hunting Dogs
It is important to recognize that dogs are able to contract brucellosis from feral swine.
32
Transmission may
occur through direct contact with the swine or by consumption of uncooked pork or scraps. Non-hunting
dogs can also become infected by contact with hunting dogs through urine or breeding. Individuals who hunt
with dogs should be encouraged not to allow their dogs to play with the animal carcass or eat raw meat.
If dogs develop symptoms consistent with brucellosis (see Additional Sources of Brucellosis Information,
Brucellosis in Animals), they should be tested for Brucella spp.
BRUCELLOSIS IN PREGNANT WOMEN
Brucellosis during pregnancy carries the risk of causing spontaneous
abortion, particularly during the first and second trimesters; therefore,
women should receive prompt medical treatment with the proper
antimicrobials.
33
The most widely recommended antimicrobial therapy
for use in pregnant women is rifampin 15-20 mg/kg per day (maximum
600-900 mg/day) for 6 weeks.
14, 17, 30, 33
Rifampin is a FDA Pregnancy
Category C drug, which indicates that there are no adequate studies or
data demonstrating risk in humans, but animal studies have shown adverse
effects on the fetus from use of this drug
.34
Also, a combination therapy
regimen of rifampin 15-20 mg/kg per day (maximum 600-900 mg/day)
plus trimethoprim-sulfamethoxazole (TMP-SMZ) 160mg-800 mg BID for
six weeks has been cited in the literature.
15, 17, 33
●
It is important to note that TMP-SMZ should not be used after 36
weeks of pregnancy because of the risk of kernicterus caused by
elevated levels of bilirubin.
14, 17
Additionally, the teratogenic potential
of many antimicrobials, including rifampin and TMP-SMZ, is unknown
in humans.
30

Brucellosis Reference Guide: Exposures, Testing, and Prevention
18
●
Information on doxycycline use during pregnancy is limited and FDA classifies it as a Pregnancy Category D
drug on the basis of data extrapolated from the use of tetracycline in humans and animals; FDA Pregnancy
Category D indicates that data have shown positive evidence of human fetal risk but benefits of drug use
may outweigh potential risks in certain situations.
34
For tetracyclines, infant dental staining, fetal growth
delays, and maternal fatty liver have been demonstrated. Reviews of studies of doxycycline use among
pregnant women have not demonstrated these findings.
35
The risk-benefit ratio for use of doxycycline must
be carefully considered if rifampin is unavailable or contraindicated.
●
Specific brucellosis treatment instructions should be made in consultation with the patient’s obstetrician.
PERSON-TO-PERSON TRANSMISSION
Neonatal Brucellosis
36–40
While neonatal brucellosis cases are rare, infection may occur through transplacental transmission of Brucella
spp. during a maternal bacteremic phase, from exposure to blood, urine, or vaginal secretions during delivery,
or through breastfeeding. The majority of documented neonatal brucellosis cases involve B. melitensis, though
cases of B. abortus have been reported as well.
●
Signs and Symptoms: Clinical manifestations typically resemble sepsis and include fever, resistance
to feeding, irritability, vomiting, jaundice, respiratory distress, pulmonary infiltrates, hypotension,
hyperbilirubinema, and thrombocytopenia. Progression of the disease state may be evidenced by
hepatomegaly, splenomegaly, and lymphadenitis. In some cases, patients may be asymptomatic or clinical
symptoms may present later in infancy.
●
Serological Testing: Information found in peer-reviewed literature suggests that Brucella spp. may be
isolated from neonatal patients with titer levels lower than 1:160.
39, 40
●
Treatment: Dual-combination antimicrobial therapy should be administered for several weeks. Duration
and dose of treatment should be made in consultation with the patient’s neonatologist or pediatrician.
●
Prevention: As Brucella bacteremia during pregnancy carries the risk of causing spontaneous abortion
(particularly during the first and second trimester) or transmission to the infant, women who are
pregnant should avoid consuming unpasteurized dairy products and engaging in high-risk occupational
activities such as contact with infected animals or administration of live attenuated Brucella vaccines.
Prompt diagnosis and treatment is essential to secure a healthy pregnancy. Women who have been
exposed to Brucella spp. or have contracted brucellosis should consult their obstetrician for PEP
and treatment options.

19
Brucellosis Reference Guide: Exposures, Testing, and Prevention
●
Information on doxycycline use during pregnancy is limited and FDA classifies it as a Pregnancy Category D
drug on the basis of data extrapolated from the use of tetracycline in humans and animals; FDA Pregnancy
Category D indicates that data have shown positive evidence of human fetal risk but benefits of drug use
may outweigh potential risks in certain situations.
34
For tetracyclines, infant dental staining, fetal growth
delays, and maternal fatty liver have been demonstrated. Reviews of studies of doxycycline use among
pregnant women have not demonstrated these findings.
35
The risk-benefit ratio for use of doxycycline must
be carefully considered if rifampin is unavailable or contraindicated.
●
Specific brucellosis treatment instructions should be made in consultation with the patient’s obstetrician.
PERSON-TO-PERSON TRANSMISSION
Neonatal Brucellosis
36–40
While neonatal brucellosis cases are rare, infection may occur through transplacental transmission of Brucella
spp. during a maternal bacteremic phase, from exposure to blood, urine, or vaginal secretions during delivery,
or through breastfeeding. The majority of documented neonatal brucellosis cases involve B. melitensis, though
cases of B. abortus have been reported as well.
●
Signs and Symptoms: Clinical manifestations typically resemble sepsis and include fever, resistance
to feeding, irritability, vomiting, jaundice, respiratory distress, pulmonary infiltrates, hypotension,
hyperbilirubinema, and thrombocytopenia. Progression of the disease state may be evidenced by
hepatomegaly, splenomegaly, and lymphadenitis. In some cases, patients may be asymptomatic or clinical
symptoms may present later in infancy.
●
Serological Testing: Information found in peer-reviewed literature suggests that Brucella spp. may be
isolated from neonatal patients with titer levels lower than 1:160.
39, 40
●
Treatment: Dual-combination antimicrobial therapy should be administered for several weeks. Duration
and dose of treatment should be made in consultation with the patient’s neonatologist or pediatrician.
●
Prevention: As Brucella bacteremia during pregnancy carries the risk of causing spontaneous abortion
(particularly during the first and second trimester) or transmission to the infant, women who are
pregnant should avoid consuming unpasteurized dairy products and engaging in high-risk occupational
activities such as contact with infected animals or administration of live attenuated Brucella vaccines.
Prompt diagnosis and treatment is essential to secure a healthy pregnancy. Women who have been
exposed to Brucella spp. or have contracted brucellosis should consult their obstetrician for PEP
and treatment options.
Sexual Transmission
41-48
Since 1966, there have been nine case reports published
in English literature that document evidence of person-to-
person transmission of brucellosis. In each of the cases,
a male patient who presented symptoms consistent with
brucellosis was thought to have transmitted Brucella spp.
to a female partner via sexual intercourse. Although rare,
it is important to recognize that sexual partners of infected
patients may be at risk for exposure to brucellosis.
Organ Donations and Blood Transfusions
While uncommon, transmission of Brucella spp. may also
occur via tissue transplantation or blood transfusions.
There are few reported cases of brucellosis caused by
blood transfusion, the earliest dating from 1955 and all
occurring outside of the United States.
49,50
There are several
reports of transmission due to transplantation, two of
which are attributed to bone marrow donation between
siblings.
51,52
In other published reports of brucellosis in
transplant recipients, it is difficult to ascertain if infection
was acquired from the transplant or through other modes
of infection.
53,54
If a patient who has undergone a recent
transfusion or transplant develops symptoms consistent
with brucellosis, the CDC Office of Blood, Organ, and
Other Tissue Safety should be contacted for assistance
with trace-back investigations.
PREVENTION
Occupational Exposures
Exposures to Brucella spp. can occur in occupational environments, which include but are not limited to:
laboratories, clinical and surgical settings, and veterinary settings. In cases involving high-risk exposures (see
Table 4 for risk assessment), post-exposure antimicrobial prophylaxis is recommended.
Clinicians should inform laboratory personnel when patient specimens are suspect or rule-outs for brucellosis.
Laboratory personnel should work with Brucella spp. in at least a class II Biological Safety Cabinet (BSC), with
proper personal protective equipment (PPE) and use of primary and secondary barriers, in compliance with
the Biosafety in Microbiological and Biomedical Laboratories (BMBL), which provides information and
recommendations on laboratory containment methods and microbiological procedures. When working with
Brucella spp. or other infectious organisms, ensure procedures are in place to minimize risk of exposure through
spills, splashes, and aerosol-generating events.
In clinical, surgical, and veterinary settings, procedures should also be performed judiciously to minimize
spills, splashes, and aerosols.
22
Depending on the types of procedures that are performed, PPE should include
adequate protection to minimize direct contact (to skin and mucous membranes) and aerosol exposures.
Examples of appropriate PPE include gloves, closed footwear, eye protection, face shield (as necessary,
depending on procedure), and respiratory protection (as necessary, depending on procedure).

Brucellosis Reference Guide: Exposures, Testing, and Prevention
20
Recreational Exposures (Hunter Safety)
Hunting wild animals carries with it the potential for risk
of exposure to infectious diseases. Certain wild animals
(e.g., feral swine, elk, moose, bison, deer, caribou) can
carry brucellosis and be a source of transmission. Predatory
animals may also be prone to brucellosis after feeding on
infected animals. When hunting, it is important to avoid
contact with animals that are found dead or are otherwise
visibly ill. Animals that appear to be healthy can still have
brucellosis; in these cases, safe field dressing techniques can
help protect hunters.
●
Use clean, sharp knives for field dressing and butchering.
●
Wear eye protection and nonporous, disposable gloves
(e.g., rubber, nitrile, or latex gloves) when handling
carcasses.
●
Avoid direct (bare skin) contact with fluid or organs
from the animal.
●
Avoid direct (bare skin) contact with hunting dogs that
may have come into contact with hunted animals.
●
After butchering, burn or bury disposable gloves and
parts of the carcass that will not be eaten.
●
Do not feed dogs with raw meat or other parts of the
carcass.
●
Wash hands as soon as possible with soap and warm
water for 20 seconds or more. Dry hands with a clean
cloth.
●
Clean all tools and reusable gloves with a disinfectant,
like dilute bleach (Follow the safety instructions on the product label).
●
Thoroughly cook meat from any animal that is known to be a possible carrier of brucellosis.
●
Be aware that freezing, smoking, drying and pickling do not kill Brucella.
This information can be found on the CDC Hunter Safety web feature.
Travel to Endemic Areas
Brucellosis is endemic in many parts of the world. High-risk areas include: Mexico, South and Central America,
Eastern Europe, Asia, Africa, the Caribbean, the Middle East, and the Mediterranean Basin (Portugal, Spain,
Southern France, Italy, Greece, Turkey, and North Africa). When traveling to these areas, be cautious of and
avoid contact with livestock and consumption of raw animal products. Consumption of raw or undercooked
meat, as well as raw or unpasteurized dairy products, can result in transmission of Brucella and potentially lead
to illness.
See CDC Brucellosis – Areas at Risk.

21
Brucellosis Reference Guide: Exposures, Testing, and Prevention
REFERENCES
1. Brucellosis 2010 CSTE Case Definition.
https://wwwn.cdc.gov/nndss/conditions/brucellosis/case-definition/2010/
2. Council of State and Territorial Epidemiologists. 2009, positing date. Public health reporting and
national notification for brucellosis, 09-ID-14. http://c.ymcdn.com/sites/www.cste.org/resource/
resmgr/PS/09-ID-14.pdf
3. National Select Agent Registry. Animal and Plant Health Inspection Service, Centers for Disease Control
and Prevention. http://www.selectagents.gov
4. Traxler RM, et al. 2013. Review of brucellosis cases from laboratory exposures in the United States in 2008
to 2011 and improved strategies for disease prevention. J. Clin. Microbiol. 51(9):3132–3136.
5. Pappas G, et al. 2006. Brucella as a biological weapon. Cell. Mol. Life Sci. 62:2229–2236.
6. The Laboratory Response Network—Partners in Preparedness. Centers for Disease Control and
Prevention. http://www.bt.cdc.gov/lrn/
7. National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention.
http://wwwn.cdc.gov/nndss/
8. Salata RA, Ravdin JI. 1985. Brucella species (brucellosis), p. 1283 – 1289. In Mandell GL, Douglas RG,
Bennett JE, ed. Principles and practices of infectious diseases, 2
nd
ed. John Wiley & Sons, Inc., New York.
9. Memish ZA, et al. 2002. Comparison of Brucella standard agglutination test with the ELISA IgG and IgM in
patients with Brucella bacteremia. Diag. Microbio. Infect. Dis.44:129-132.
10. Fadeel MA, Hoffmaster AR, Shi J, Pimentel G, Stoddard RA. 2011. Comparison of four commercial IgM
and IgG ELISA kits for diagnosing brucellosis. Journal of Medical Microbiology. 60:1-7.
11. Al Dahouk S, et al. 2003. Laboratory-based Diagnosis of Brucellosis -- A Review of the Literature. Clin.
Lab. 49:577-589.
12. Araj GF, et al. 2005. Evaluation of the PANBIO Brucella Immunoglobulin G (IgG) and IgM Enzyme-Linked
Immunosorbent Assays for Diagnosis of Human Brucellosis. Clin. Diagn. Lab. Immunol. 12(11): 1334-
1335. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=16275951
13. Red Book
®
: 2009 Report of the Committee on Infectious Diseases - 28th Ed.
14. Al-Tawfiq JA. 2008. Therapeutic options for human brucellosis. Expert Rev. Anti Infect. Ther. 6:109–120.
15. Laboratory-Acquired Brucellosis --- Indiana and Minnesota http://www.cdc.gov/mmwr/preview/
mmwrhtml/mm5702a3.htm
16. Ashford DA, di Pietra J, Lingappa J, Woods C, Noll H, Neville B, Weyant R, Bragg SL, Spiegel RA, Tappero
J, Perkins BA. 2004. Adverse events in humans associated with accidental exposure to the livestock
brucellosis vaccine RB51. Vaccine 22:3435–3439.
17. Solera J, Martinez-Alfaro E, Espinosa A, Castillejos ML, Geijo P, Rodriguez-Zapata M. 1998. Multivariate
model for predicting relapse in human brucellosis. J. Infect. 1998; 36: 85–92.
18. Siegel JA, et al. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in
Healthcare Settings. http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf
19. Kiel FW, Khan MY. 1990. Brucellosis among Hospital Employees in Saudi Arabia. Infect. Control Hosp.
Epidemiol. 14(5): 268–272.
20. Poulou A, et al. 2006. A rare case of Brucella melitensis infection in an obstetrician during the delivery of a
transplacentally infected infant. J. Infect. 53:39–41.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
22
21. Mesner, et al. 2007.The many faces of human-to-human transmission of brucellosis: congenital infection
and outbreak of nosocomial disease related to an unrecognized clinical case.
22. Nichols M, Thompson D, Carothers JT, Klauber J, Stoddard RA, Traxler RM, Benoit TJ, Guerra MA. 2013.
Brucella abortus exposure during an orthopedic surgical procedure—New Mexico, 2010.
23. Wong KC, Leung KS. 2004.Transmission and prevention of occupational infections in orthopaedic
surgeons. J. Bone Joint Surg. Am. 86-A(5): 1065–1076.
24. CDC. 1998. Human exposure to Brucella abortus strain RB51--Kansas, 1997. MMWR.
http://www.cdc.gov/mmwr/preview/mmwrhtml/00051495.htm
25. Blasco GM, Diaz R. 1993. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. The Lancet.
342:805.
26. Wallach JC, Ferrero MC, Victoria Delpino M, Fossati CA, Baldi PC. 2008. Occupational infection due to
Brucella abortus S19 among workers involved in vaccine production in Argentine. Clin Microbiol Infect. 14:
805–807.
27. Glynn MK, Lynn TV. 2008. Zoonosis Update: Brucellosis. JAVMA 233(6): 900–908.
28. CDC. 2012. Human exposures to marine Brucella isolated from a harbor porpoise—Maine 2012. MMWR.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6125a2.htm
29. NMFS Marine Mammal Health and Stranding Response Program. 2012. Revised Interim Marine Mammal
Brucella Specific Biosafety Guidelines for the National Marine Mammal Stranding Network.
30. World Health Organization. Brucellosis in humans and animals. 2007
http://www.who.int/csr/resources/publications/Brucellosis.pdf
31. CDC. 2009. Brucella suis infection associated with feral swine hunting—three states, 2007-2008. MMWR.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5822a3.htm
32. Ramamoorthy S, et al. 2011. Brucella suis infection in dogs, Georgia, USA [letter]. Emerg Infect Dis.
http://dx.doi.org/10.3201/eid1712.111127
33. Khan MY, Mah MW, Memish ZA. 2001. Brucellosis in pregnant women. Clin Infect Dis. 32: 1172-1177.
34. U.S. Department of Health and Human Services: Chemical Hazards Emergency Medical Management.
FDA Pregnancy Categories. http://chemm.nlm.nih.gov/pregnancycategories.htm.
35. Nahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: What is and is not known
about teratogenic and toxic risks. Obstet Gynecol. 2006;107:1120–38.
36. Yagupsky P. 2010. Neonatal brucellosis: rare and preventable. Annals of Tropical Paediatrics. 30: 177–179.
37. Giannacopoulos I, Eliopoulos MI, Ziambraras T, et al. 2002. Transplacentally transmitted congenital
brucellosis due to Brucella abortus. J Infect. 45: 209–210.
38. American Academy of Pediatrics. Brucellosis. In: Pickering LK, ed. Red Book: 2003 Report of the
Committee on Infectious Diseases. 26
th
ed. American Academy of Pediatrics; 203: 222–224.
39. Al Eissa YA, Al Zamil F, Al Mugeiren M, Al Rasheed S, Al Sanie A, Al Mayzad A. 1991. Childhood
brucellosis: a desceptive infectious disease. Scan J Infect Dis 23: 129–133.
40. Mosayebi Z, Movahedian AH, Ghayomi A, Kazemi B. 2005. Congenital brucellosis in a preterm neonate.
Indian Pediatrics. 42:599–601.
41. Goossens H, Marcelis L, Dekeyser P, Butzler JP. 1983. Brucella melitensis: person-to-person transmission?
Lancet. 1:773.

23
Brucellosis Reference Guide: Exposures, Testing, and Prevention
42. Stantic-Pavlinic M, Cec V, Megle J. 1983. Brucellosis in spouses and the possibility of interhuman
infection. Infection. 11:313 –314.
43. Ruben B, Band JD, Wong P, Colville J. 1991. Person-to-person transmission of Brucella melitensis. Lancet.
337:14 –15.
44. Lindberg J, Larsson P. 1991. Transmission of Brucella melitensis. Lancet. 337:848–849.
45. Mantur BG, Mangalgi SS, Mulimani M. 1996. Brucella melitensis—a sexually transmissible agent? Lancet.
347:1763.
46. Thalhammer F, Eberl G, Kopetzki-Kogler U. 1998. Unusual route of transmission for Brucella abortus. Clin
Infect Dis. 26:763–764.
47. Kato Y, Masuda G, Ichiro I, Imamura A, Ajisawa A, Megishi M. 2007. Brucellosis in a returned traveler
and his wife: Probable person-to-person transmission of Brucella melitensis. Journal of Travel Medicine.
14(5): 1195–1982.
48. Meltzer E, Sidi Y, Smolen G, Banai M, Bardenstein S, Schwartz E. 2010. Sexually transmitted brucellosis in
humans. Clin Infect Dis. 51(2): e12–e15.
49. Wood EE. 1955. Brucellosis as a hazard of blood transfusion. Br Med J. 1(4904): 27–28.
50. Doganay M, Aygen B, and Esel D. 2001. Brucellosis due to blood transfusion. J Hospital Infect.
49(2):151-2.
51. Neparstek E, Block CS, and Slavin S. 1982. Lancet, Mar 6;1(8271):574-5.
52. Ertem M, Kurekci AE, Aysev D, Unal E, and Ikinciogullari A. Brucellosis transmitted by bone marrow
transplantation. 2000. Bone Marrow Transplantation. 26:225-6.
53. Einollahi B, Hamedanizadeh AK, Alavian SM. 2003. Brucellosis arthritis—a rare complication of renal
transplantation: A case report. Transplantation Proceedings. 35:2698.
54. Yousif B, Nelson J. 2001. Am J Nephrol. 21:66.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
24
ADDITIONAL SOURCES OF BRUCELLOSIS INFORMATION:
●
CDC Brucellosis webpage: http://www.cdc.gov/brucellosis
Treatment of Brucellosis:
●
Ariza J et al. 2007. Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina
Recommendations. PLoS Med. 4(12): e317.
http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0040317
●
Al-Tawfiq JA. 2008. Therapeutic options for human brucellosis. Expert Rev Anti Infect Ther. 6(1): 109-120.
http://www.ncbi.nlm.nih.gov/pubmed/18251668
●
Solera J. 2010. Update on brucellosis: therapeutic challenges. Intl J Antimicrob Agent. 36S, S18–S20.
http://www.ncbi.nlm.nih.gov/pubmed/20692127
Laboratory Exposures:
●
Laboratory-Acquired Brucellosis --- Indiana and Minnesota
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5702a3.htm
RB51 Exposures:
●
Update: Potential Exposures to Attenuated Vaccine Strain Brucella abortus RB51 During a Laboratory
Proficiency Test --- United States and Canada, 2007
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5702a2.htm
●
Ashford et al. (2004) Adverse events in humans associated with accidental exposure to the livestock
brucellosis vaccine RB51. Vaccine 22(25-26): 3435–3439.
Rev-1 Exposures:
●
Immunization with viable Brucella organisms: Results of a safety test in humans. 1962. Bull. Wld Hlth
Org. 22: 409–419.
http://whqlibdoc.who.int/bulletin/1962/Vol26/Vol26-No3/bulletin_1962_26(3)_409-419.pdf
Brucellosis in Feral Swine Hunters:
●
Brucella suis Infection Associated with Feral Swine Hunting --- Three States, 2007–2008
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5822a3.htm
●
Feral Swine Hunter Brochure http://www.cdc.gov/brucellosis/pdf/brucellosis_and_hoghunters.pdf
●
Davis J. and Ivey M. 2011. “Kill Feral Hogs, But Handle Them Carefully For Your Health and Dogs.”
Georgia Outdoor News. http://www.gon.com/article.php?id=2866
Brucellosis in Animals:
●
Shin S and Carmichael LE. 1999. Canine Brucellosis Caused by Brucella canis. Recent Advances in Canine
Infectious Diseases. Avail at: International Veterinary Information Service:
http://www.ivis.org/advances/Infect_Dis_Carmichael/shin/ivis.pdf
●
WHO Brucellosis in humans and animals 2006.
http://www.who.int/entity/csr/resources/publications/deliberate/WHO_CDS_EPR_2006_7/en
●
Compendium of Measures to Prevent Disease Associated with Animals in Public Settings, 2009. National
Association of State Public Health Veterinarians, Inc. (NASPHV)
http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5805a1.htm

25
Brucellosis Reference Guide: Exposures, Testing, and Prevention
●
Canine Brucellosis
Shin S and Carmichael LE. 1999. Canine Brucellosis Caused by Brucella canis. Recent Advances in
Canine Infectious Diseases. Avail at: International Veterinary Information Service:
http://www.ivis.org/advances/Infect_Dis_Carmichael/shin/ivis.pdf
Center for Food Security and Public Health, Iowa State University. Canine Brucellosis: Brucella canis.
http://www.cfsph.iastate.edu/Factsheets/pdfs/brucellosis_canis.pdf
●
Marine Mammal Brucellosis
American Association of Zoo Veterinarians
http://c.ymcdn.com/sites/www.aazv.org/resource/resmgr/imported/marine%20mammal%20
Brucella.pdf
CDC Marine Mammal Brucella Genotype Associated with Zoonotic Infection
http://wwwnc.cdc.gov/eid/article/14/3/07-0829_article.htm
Center for Food Security and Public Health, Iowa State University
http://www.cfsph.iastate.edu/Factsheets/pdfs/brucellosis_marine.pdf
National Oceanic and Atmospheric Administration (NOAA) Fisheries
http://www.nmfs.noaa.gov/pr/health/mmume/midatlantic2013/brucella_factsheet2013.pdf

Brucellosis Reference Guide: Exposures, Testing, and Prevention
26
APPENDIX 1: SPECIMEN SUBMISSION
Submission of Serum for Brucella Serology
Zoonotic and Select Agent Laboratory, Bacterial Special Pathogens Branch
1. Acute- and convalescent-phase serum specimens that are shipped together are preferred.
2. Please send serum, not whole blood.
3. Serum should be sent in Sarstedt 2ml micro tubewith an O-ring in the lid (ref #72.694.006). The O-ring
helps to prevent leaking or drying of sample.
4. Refrigeration during shipment is preferred. If the sample was previously frozen please ship frozen.
A. If this is a large-scale exposure (~15 samples), please ship thawed.
5. Specimens are supposed to go first to the physician’s state health laboratory (SHL), and if the SHL does
not perform the test requested, the SHL will forward the specimens to CDC. If the physician knows that
the SHL cannot perform the test and the SHL has given the physician permission to by-pass it, specimens
can be sent directly to CDC (to DASH) at the following address:
Centers for Disease Control & Prevention
Data & Specimen Handling Section (DASH)
Mailstop G12
1600 Clifton Rd NE
Atlanta, GA 30329-4027
6. CDC Form 50.34 should accompany the specimens; the form is included.
7. When filling out the form, the more complete the form the better. The minimal information should
include the following: test requested (Brucella serology), submitter’s name and address, patient’s name/
sex/ DOB, type of specimen, collection dates, date of onset of symptoms, and history of travel, water,
animal, or other suspected exposures.
Please contact if you have additional questions regarding Brucella serology:
Dr. Robyn Stoddard
Tel: (404) 639–2053
Fax: (404) 639–3022
Renee Galloway
Tel: (404) 639–5461
Fax: (404) 639–3022

27
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Renee Galloway
Tel: (404) 639–5461
Fax: (404) 639–3022
Submission of Brucella Isolate(s) Zoonotic and Select Agent Laboratory,
Bacterial Special Pathogens Branch
AS A DIAGNOSTIC SAMPLE: Any suspected Brucella isolate that requires confirmatory testing in our lab:
1. Fill out the CDC 50.34 DASH form
●
When filling out the CDC 50.34 form, the more complete the form, the better.
●
The minimal information should include the following: suspected Brucella species, submitter’s name and
address, patient’s name/sex/DOB, type of specimen, collection dates, date of onset of symptoms, and
history of travel, water, animal, or other suspected exposures (and any other pertinent epidemiological
data).
2. Send the culture on an agar slant (not a plate) directly to CDC via DASH to:
Centers for Disease Control & Prevention
Data & Specimen Handling Section (DASH)
Mailstop G12
1600 Clifton Rd NE
Atlanta, GA 30329-4027
ATTN: Rebekah Tiller or Elke Saile
AS A SELECT AGENT: Any B. melitensis, B. suis or B. abortus isolate that has been previously identified/
confirmed by another lab that has prepared and submitted a “Form 4: Identification of a Select Agent or Toxin.”
1. Because CDC is the recipient lab, we will request certain information from the sending lab, so that we
can complete the “Form 2: Request to Transfer Select Agents and Toxins” and submit to DSAT for approval to
transfer.
2. We will both be notified by DSAT of the approval, upon which we have 30 days to complete the transfer.
At this time, we will send you an e-mail with detailed information on the shipping of the strain(s) to our
lab.
3. We do not receive shipments after business hours or on the weekends so it is best to ensure your
shipment arrives Monday-Thursday
4. Please send the culture on an agar slant (not a plate).
5. The shipment may be addressed to:
Rebekah Tiller or Elke Saile
1600 Clifton Rd, NE
Bldg 17 Room 2021
Atlanta, GA 30329-4027
6. Brucella canis is NOT a select agent. Therefore, you can send a slant culture directly to CDC via DASH
using the CDC 50.34 DASH form only.
Please contact us if you have additional questions regarding isolate submission
or Brucella molecular detection:
Rebekah Tiller
Tel: (404) 639–4507
Fax: (404) 639–3023
Elke Saile
Tel: (404) 639–0716
Fax: (404) 639–3023

Brucellosis Reference Guide: Exposures, Testing, and Prevention
28
APPENDIX 2: POST-EXPOSURE MONITORING
Follow-up of Brucella occupational exposure
(The following should only be used as a guide by healthcare professionals who are assessing an exposure.)
Name: _______________________________ Title/Occupation: __________________________
Exposure Date(s): ____/____/_______ -- ____/____/_______
Years of experience (as a clinician/veterinarian/lab technician): __________
Years working in this facility: _________
Sex: ___M ___F Pregnant? __Yes __No __Unknown
Based on CDC risk assessment guidelines, what risk level applies to this employee?
____ High ____ Low ____ None
Serologic Monitoring
Note: Individuals should be monitored for an antibody response to Brucella sp. depending on their level of risk of exposure.
Serological testing is not available for B. canis or B. abortus RB51; it is available for S19 and Rev-1 exposures.
The Week 0 specimen should be drawn as close to the last date of exposure as possible. A banked serum sample may substitute
for the Week 0 sample. The following samples in the series should be drawn at the specified week after the exposure, not from
the time the exposure was identified. For example, if a baseline sample is drawn at Week 2 following the exposure incident, the
second draw should take place at Week 6 and not Week 8 (six weeks from the baseline draw).
If an exposed worker seroconverts, please contact your state health department immediately. Brucellosis is a
reportable disease, and the individual will need to undergo brucellosis treatment and confirmatory testing.

29
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Post-Exposure Prophylaxis Regimen
If the worker was recommended to take PEP, please ask the individual the following questions:
1. Which antibiotics were recommended to you? Please mark all that apply.
Unsure/Don’t Know Doxycycline Rifampin TMP-SMZ/Bactrim
Other (Antibiotic Name): _________________________________________
2A. Did you start taking the medication? Yes No
2B. If yes, when? ____/____/____ Unsure/Don’t Know
2C. If no, why didn’t you start? Complete, then skip to Section H
Did not feel that I was at risk for becoming sick Pregnancy
Side effects of antibiotics
Other: __________________________________________________
3A. Did you miss any doses of the antibiotics? Note this indicates doses not days missed.
Yes No If “No”, skip to Q4
3B. Which antibiotics did you miss doses for and how many total doses do you think you missed?
Unsure/Don’t Know Doxycycline (Doses):____________ Rifampin (Doses):_____________
TMP-SMZ/Bactrim (Doses):_________ Other: (Antibiotic Name): ___________________________
(Doses):_____________
3C. Why did you miss doses of the antibiotics? Side effects (adverse events) Forgot to take
Other:____________________________________________________________________________
4A. Did you complete the full 3-week course of antibiotics, as recommended? Yes No
4B. If no, why didn’t you finish?
Did not feel that I was at risk Side effects Switched antibiotics Pregnancy
Other: ____________________________________________________________________________
5A. Did you have any side effects that were caused by the medication?
Yes No Unsure/Don’t Know
If “Yes,” please fill out the table below. Rate the severity of each reaction on a scale of 1 to 5, with 5 being most severe.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
30
5B. If yes, did any of these reactions cause you to miss work?
Yes How much work did you miss? _______ Hours Days
No
Symptom Monitoring
All exposed individuals, regardless of risk status, should be monitored for the development of symptoms.
Arrange for regular (e.g., weekly) active surveillance for febrile illness among all workers exposed to Brucella
isolates for six months after last exposure. Broader symptoms of brucellosis should be passively monitored for
six months from the last exposure.
At each regular appointment, ask the worker if he/she has experienced any of the following signs or symptoms.
Mark if the worker has had any of the following signs or symptoms not attributed to a pre-existing medical
condition. If the worker has experienced any of these, please indicate the date the sign or symptom started.
If you choose to use this table, enter the date the worker is seen by Occupational Health (OH). It is up to
the OH personnel to decide the frequency of surveillance, whether it is daily, weekly, or a combination for six
months following the exposure. Place a check mark in the box if a worker has experienced a specific symptom
since the last time he or she was seen by the OH.
Use of the Symptom Monitoring table on the following page is optional.
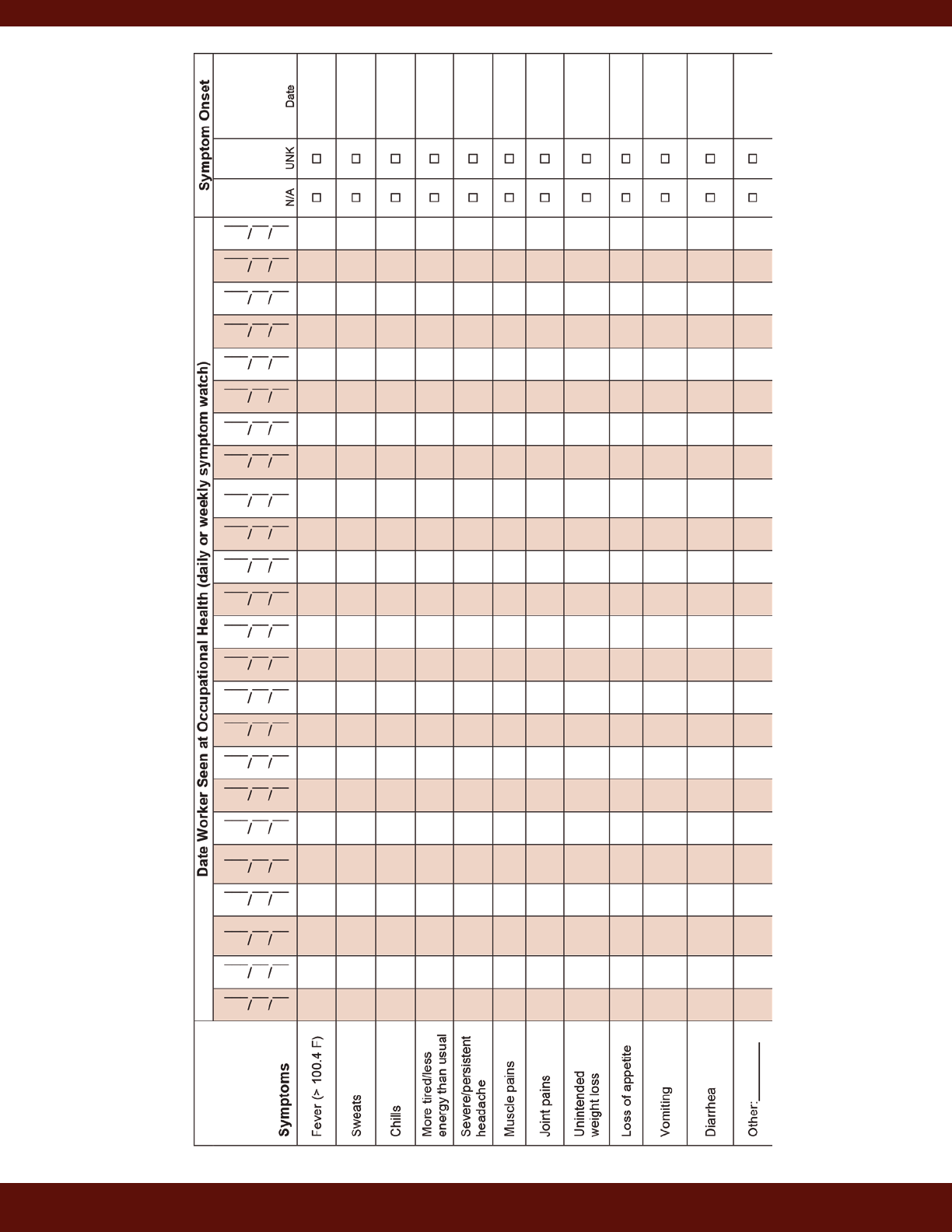
31
Brucellosis Reference Guide: Exposures, Testing, and Prevention
Signs and Symptoms of Brucellosis

Brucellosis Reference Guide: Exposures, Testing, and Prevention
32
APPENDIX 3: INTERIM MARINE MAMMAL BIOSAFETY GUIDELINES
EXCERPT FROM: Revised Interim Marine Mammal Brucella Specific Biosafety Guidelines for the National
Marine Mammal Stranding Network:
Below are current recommendations from the NMFS Marine Mammal Health and Stranding Response
Program (MMHSRP) to protect the health of stranding network personnel when handling cetaceans or
pinnipeds suspected of being infected with Brucella.
A. High Risk Categories of Animals for Suspect Brucella Infection in Marine Mammals:
(Use these case definitions when assessing risk and PPE requirements)
●
Cetaceans:
Any Species – Perinates/Neonates
Any Species – Juveniles/Sub-adults/Adults with skin or bony lesions
Any Species – Pregnant Females*
●
Pinnipeds
Any Species – Pregnant Females*
Harbor Seals – Weaned pups, Yearlings and Sub-adults (with active lungworm infections)**
*Also are a high risk age class for Q Fever, Leptospirosis infections
**Based upon studies in the Pacific NW
B. Personal Protective Equipment (PPE) for Handling Animals with Suspect Infectious Diseases
including Brucella
All personnel are recommended to wear appropriate PPE, see list below, personnel can choose to wear
more PPE than the minimum recommendations
●
Minimum Recommended PPE for Collecting Stranded Live Animals from the Field
Gloves (disposable or can be disinfected)
Closed footwear (that can be disinfected)
Eye protection (goggles, glasses, etc.) if feasible
●
Minimum Recommended PPE for Rehabilitating Stranded Cetaceans – For Cetaceans with Skin Lesions
(not old scars):
No persons with open cuts or wounds should handle the animal
Long-sleeved rash guards and long pants or shorts and/or full wetsuits/skins
Gloves (disposable or dive gloves)
Dive boots/footwear
Eye protection (goggles, glasses, etc.) if feasible
●
Minimum Recommended PPE for Collecting Dead Animals from the Field
Gloves (disposable or can be disinfected)
Closed footwear (that can be disinfected)
Face shields if carcass is open, has open wounds/skin lesions or if placenta/umbilicus is present
(when feasible dependent upon field conditions)

33
Brucellosis Reference Guide: Exposures, Testing, and Prevention
●
Minimum Recommended PPE for Necropsy (field or laboratory)
Gloves (disposable, preferably double-glove during necropsy)
Rubber boots or closed footwear (that can be disinfected)
Face shields (for field necropsies use if appropriate environmental conditions)
Tyvek suits, slickers, other coverall type clothing, waterproof aprons, and/or full wetsuits/skins as
appropriate for environmental conditions including temperature and necropsy location
●
Special Necropsy PPE for Procedures that Create Aerosols (Stryker Saw/High Pressure Hose Cleaning).
Wear Minimum Recommended Necropsy PPE as listed above with additional recommendations:
Respiratory protection such as a NIOSH-certified N95 (or greater) filtering facepiece respiratory or
equivalent is recommended. All respirator users should be fit-tested before use and respirators should
be used within the context of a complete respiratory program that meets the requirements in the
OSHA Respiratory Protection Standard (29 CFR 1910.134)
See CDC Safe Work Practices Guidelines-Appendix I for more information and
http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=9780&p_table=STANDARDS
●
General Protective Guidelines—In general, precautionary measures to prevent skin and mucous membrane
(eyes, nose, and mouth) exposure to fluids, excretions, and tissues from stranded marine mammals or
aborted fetuses can decrease risk of infection. The following recommendations are listed in order of
importance.
Hand hygiene. Proper hand washing is the most important element of infection control. Wearing
gloves does not replace the need for proper hand washing with soap and water, or alcohol-based hand
sanitizer if soap and water are not available.
When appropriate for environmental conditions wear protective clothing during necropsy,
including coveralls or coat, rubber or plastic apron, or rain slicker. Impermeable outer garments are
recommended if potential exists for clothing to become soaked with animal fluids. Waterproof boots
that can be cleaned and disinfected are recommended. Disinfect boots after each use.
Outer clothes worn when working with animals or tissues should be cleaned at work when feasible.
Disposable items should not be reused, and wash and dry soiled clothing separately from other items.
Disposable gloves are recommended when handling potentially infected animals, tissues, fluids, or
excretions.
Clean and treat cuts and scratches with an antiseptic and cover with a bandage. Report injuries to
your supervisor and document appropriate medical treatment.
A face shield that covers the entire face is recommended to be worn during all necropsy procedures
and especially if splashes or sprays are anticipated. Full-face plastic shields are preferred over goggles
as they provide additional protection for the mouth and nose. Should any potentially infectious
material enter the eye, the eye should be flushed for at least 10 minutes with water under clean or
aseptic conditions. Exposure should be documented and prompt medical attention is recommended.
Activities that generate dust or aerosols should be avoided as much as possible to limit the potential
for respiratory exposure. Wet mopping, High-Efficiency Particulate Air (HEPA) vacuums, and avoiding
the use of high pressure water sprayers for cleaning is recommended. PPE as described above are
recommended to be worn during necropsy and cleaning activities.
If feasible, staff should consider participating in a medical surveillance program. Baseline serology
should be considered for new staff before starting work.

Brucellosis Reference Guide: Exposures, Testing, and Prevention
34
People under 18 years of age, pregnant women and people with weakened immune systems are
recommended to be excluded from handling potentially infected animals or tissues because these
people are at higher risk for infection.

35
Brucellosis Reference Guide: Exposures, Testing, and Prevention

CS275573
